Modifications of Protein-Bound Substrates by Trans-Acting Enzymes in Natural Products Biosynthesis
- PMID: 38386898
- PMCID: PMC11021167
- DOI: 10.1002/cbic.202400056
Modifications of Protein-Bound Substrates by Trans-Acting Enzymes in Natural Products Biosynthesis
Abstract
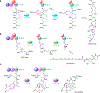
Enzymatic modifications of small molecules are a common phenomenon in natural product biosynthesis, leading to the production of diverse bioactive compounds. In polyketide biosynthesis, modifications commonly take place after the completion of the polyketide backbone assembly by the polyketide synthases and the mature products are released from the acyl-carrier protein (ACP). However, exceptions to this rule appear to be widespread, as on-line hydroxylation, methyl transfer, and cyclization during polyketide assembly process are common, particularly in trans-AT PKS systems. Many of these modifications are catalyzed by specific domains within the modular PKS systems. However, several of the on-line modifications are catalyzed by stand-alone proteins. Those include the on-line Baeyer-Villiger oxidation, α-hydroxylation, halogenation, epoxidation, and methyl esterification during polyketide assembly, dehydrogenation of ACP-bound short fatty acids by acyl-CoA dehydrogenase-like enzymes, and glycosylation of ACP-bound intermediates by discrete glycosyltransferase enzymes. This review article highlights some of these trans-acting proteins that catalyze enzymatic modifications of ACP-bound small molecules in natural product biosynthesis.
Keywords: acyl carrier protein; on-line modification; polyketide synthase; tailoring reaction; trans-acting enzyme.
© 2024 Wiley‐VCH GmbH.
Figures





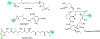




Similar articles
-
The structure of full-length AFPK supports the ACP linker in a role that regulates iterative polyketide and fatty acid assembly.Proc Natl Acad Sci U S A. 2025 Feb 11;122(6):e2419884122. doi: 10.1073/pnas.2419884122. Epub 2025 Feb 6. Proc Natl Acad Sci U S A. 2025. PMID: 39913209 Free PMC article.
-
Divergent Tandem Acyl Carrier Proteins Necessitate In-Series Polyketide Processing in the Leinamycin Family.Angew Chem Int Ed Engl. 2025 Jan 10;64(2):e202414165. doi: 10.1002/anie.202414165. Epub 2024 Nov 6. Angew Chem Int Ed Engl. 2025. PMID: 39324406 Free PMC article.
-
Discovery of Tricyclic Aromatic Polyketides Reveals Hidden Chain-Length Flexibility in Type II Polyketide Synthases.Int J Mol Sci. 2025 Aug 13;26(16):7801. doi: 10.3390/ijms26167801. Int J Mol Sci. 2025. PMID: 40869122 Free PMC article.
-
Structural insights and rational engineering strategies for modular polyketide synthases: A review.Int J Biol Macromol. 2025 Aug;319(Pt 3):145299. doi: 10.1016/j.ijbiomac.2025.145299. Epub 2025 Jun 16. Int J Biol Macromol. 2025. PMID: 40533022 Review.
-
Genome-based discovery of polyketides generated by trans-acyltransferase polyketide synthases.Methods Enzymol. 2025;717:317-347. doi: 10.1016/bs.mie.2025.03.002. Epub 2025 Apr 22. Methods Enzymol. 2025. PMID: 40651830 Review.
References
-
- a) Walsh CT, Chen H, Keating TA, Hubbard BK, Losey HC, Luo L, Marshall CG, Miller DA, Patel HM, Curr Opin Chem Biol 2001, 5, 525–534; - PubMed
- b) Xu J, Wan E, Kim CJ, Floss HG, Mahmud T, Microbiology 2005, 151, 2515–2528; - PubMed
- c) Rix U, Fischer C, Remsing LL, Rohr J, Nat Prod Rep 2002, 19, 542–580. - PubMed
-
- Staunton J, Wilkinson B, Chem Rev 1997, 97, 2611–2630. - PubMed
-
- a) Luhavaya H, Dias MV, Williams SR, Hong H, de Oliveira LG, Leadlay PF, Angew Chem Int Ed Engl 2015, 54, 13622–13625; - PMC - PubMed
- b) Hiratsuka T, Suzuki H, Kariya R, Seo T, Minami A, Oikawa H, Angew Chem Int Ed Engl 2014, 53, 5423–5426; - PubMed
- c) Sato M, Yagishita F, Mino T, Uchiyama N, Patel A, Chooi YH, Goda Y, Xu W, Noguchi H, Yamamoto T, Hotta K, Houk KN, Tang Y, Watanabe K, ChemBioChem 2015, 16, 2294–2298; - PMC - PubMed
- d) Sundaram S, Hertweck C, Curr Opin Chem Biol 2016, 31, 82–94; - PubMed
- e) Pang B, Wang M, Liu W, Nat Prod Rep 2016, 33, 162–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

