SMART-ESAS: Smartphone Monitoring and Assessment in Real Time of Edmonton Symptom Assessment System Scores for Patients With Cancer
- PMID: 38386957
- PMCID: PMC10898676
- DOI: 10.1200/GO.23.00447
SMART-ESAS: Smartphone Monitoring and Assessment in Real Time of Edmonton Symptom Assessment System Scores for Patients With Cancer
Abstract
Purpose: Serial patient-reported outcome (PRO) measurements in clinical practice are associated with a better quality of life and survival. Recording electronic PROs using smartphones is an efficient way to implement this. We aimed to assess the feasibility of the electronically filled Edmonton Symptom Assessment System (e-ESAS) scale in the lower-middle-income country (LMIC) setting.
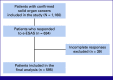
Methods: Baseline clinical features and conventional paper-based ESAS (p-ESAS) were collected in newly diagnosed patients with solid organ tumors. Text message link was sent to these patients for filling e-ESAS. ESAS was categorized into physical, psychological, and total symptom domains. Scores were divided into none to mild (0-3) and moderate to severe (4-10). Intraclass correlation coefficients (ICCs) were used to determine the correlation between p-ESAS and e-ESAS. Multivariable logistic regression was used to identify independent factors affecting symptom burden.
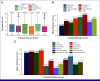
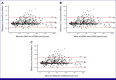
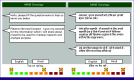
Results: Of 1,160 participants who filled out p-ESAS, 595 completed both e-ESAS and p-ESAS questionnaires and were included in the final analysis. Moderate to severe physical, psychological, and total symptom scores were seen in 39.8%, 40%, and 39% of participants. Tiredness and anxiety were the most common physical and psychological symptoms, respectively. ICCs between the p-ESAS and e-ESAS varied between 0.75 and 0.9. Total symptom scores were independently predicted by metastatic disease (odds ratio [OR], 1.83; 95% CI, 1.26 to 2.67; P = .001) and a higher level of education (OR, 0.42; 95% CI, 0.25 to 0.72; P = .001).
Conclusion: Paper-based and electronically filled ESASs have good intraobserver reliability across individual symptoms and domain scores in a representative cohort at a tertiary care institute in the LMIC. This may help us incorporate e-ESAS in routine clinical care in the real-world setting with financial, infrastructural, and manpower limitations.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
Similar articles
-
Validation of the Edmonton Symptom Assessment System in Korean patients with cancer.J Pain Symptom Manage. 2013 Dec;46(6):947-56. doi: 10.1016/j.jpainsymman.2013.01.012. Epub 2013 Apr 28. J Pain Symptom Manage. 2013. PMID: 23628516 Free PMC article.
-
Province-Wide Analysis of Patient-Reported Outcomes for Stage IV Non-Small Cell Lung Cancer.Oncologist. 2021 Oct;26(10):e1800-e1811. doi: 10.1002/onco.13890. Epub 2021 Jul 17. Oncologist. 2021. PMID: 34216415 Free PMC article.
-
Edmonton symptom assessment scale: Italian validation in two palliative care settings.Support Care Cancer. 2006 Jan;14(1):30-7. doi: 10.1007/s00520-005-0834-3. Epub 2005 Jun 4. Support Care Cancer. 2006. PMID: 15937688
-
Prediction of breast cancer-related outcomes with the Edmonton Symptom Assessment Scale: A literature review.Support Care Cancer. 2021 Feb;29(2):595-603. doi: 10.1007/s00520-020-05755-9. Epub 2020 Sep 11. Support Care Cancer. 2021. PMID: 32918128 Review.
-
The psychometric properties of cancer multisymptom assessment instruments: a clinical review.Support Care Cancer. 2015 Jul;23(7):2189-202. doi: 10.1007/s00520-015-2732-7. Epub 2015 Apr 19. Support Care Cancer. 2015. PMID: 25894883 Review.
Cited by
-
Engagement With Daily Symptom Reporting, Passive Smartphone Sensing, and Wearable Device Data Collection During Chemotherapy: Longitudinal Observational Study.JMIR Cancer. 2024 Dec 10;10:e57347. doi: 10.2196/57347. JMIR Cancer. 2024. PMID: 39656513 Free PMC article.
References
-
- Sanson-Fisher R, Girgis A, Boyes A, et al. : The unmet supportive care needs of patients with cancer. Supportive Care Review Group. Cancer 88:226-237, 2000 - PubMed
-
- Howell D, Molloy S, Wilkinson K, et al. : Patient-reported outcomes in routine cancer clinical practice: A scoping review of use, impact on health outcomes, and implementation factors. Ann Oncol 26:1846-1858, 2015 - PubMed
-
- US Department of Health and Human Services FDA Center for Drug Evaluation and Research; US Department of Health and Human Services FDA Center for Biologics Evaluation and Research; US Department of Health and Human Services FDA Center for Devices and Radiological Health : Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health Qual Life Outcomes 4:79, 2006 - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

