Long-term treatment with rilzabrutinib in patients with immune thrombocytopenia
- PMID: 38386978
- PMCID: PMC10997915
- DOI: 10.1182/bloodadvances.2023012044
Long-term treatment with rilzabrutinib in patients with immune thrombocytopenia
Abstract
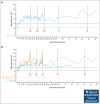
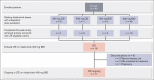
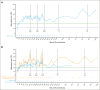
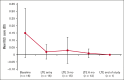
Immune thrombocytopenia (ITP) is an autoimmune disease associated with autoantibody-mediated platelet destruction and impaired platelet production, resulting in thrombocytopenia and a predisposition to bleeding. The ongoing, global phase 1/2 study showed that rilzabrutinib, a Bruton tyrosine kinase inhibitor specifically developed to treat autoimmune disorders, could be an efficacious and well-tolerated treatment for ITP. Clinical activity, durability of response, and safety were evaluated in 16 responding patients who continued rilzabrutinib 400 mg twice daily in the long-term extension (LTE) study. At LTE entry, the median platelet count was 87 × 109/L in all patients, 68 × 109/L in those who had rilzabrutinib monotherapy (n = 5), and 156 × 109/L in patients who received concomitant ITP medication (thrombopoietin-receptor agonists and/or corticosteroids, n = 11). At a median duration of treatment of 478 days (range, 303-764), 11 of 16 patients (69%) continued to receive rilzabrutinib. A platelet count of ≥50 × 109/L was reported in 93% of patients for more than half of their monthly visits. The median percentage of LTE weeks with platelet counts ≥30 × 109/L and ≥50 × 109/L was 100% and 88%, respectively. Five patients discontinued concomitant ITP therapy and maintained median platelet counts of 106 × 109/L at 3 to 6 months after stopping concomitant ITP therapy. Adverse events related to treatment were grade 1 or 2 and transient, with no bleeding, thrombotic, or serious adverse events. With continued rilzabrutinib treatment in the LTE, platelet responses were durable and stable over time with no new safety signals. This trial is registered at www.clinicaltrials.gov as #NCT03395210 and www.clinicaltrialsregister.eu as EudraCT 2017-004012-19.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: D.J.K. reports consultancy fees and research funding from Actelion (Syntimmune), Agios, Alnylam, Amgen, Argenx, BioCryst, Bristol Myers Squibb (BMS), Immunovant, Principia, Protalex, Rigel, Takeda (Bioverativ), and Union Chimique Belge (UCB); research funding from Kezar; and consultancy from Caremark, Cellphire, Cellularity, CRICO, Daiichi Sankyo, Dova, Genzyme, Hengrui, Incyte, Kyowa Kirin, Merck Sharp & Dohme, Momenta, Novartis, Pfizer, Platelet Biogenesis, Platelet Disorder Support Association, Sanofi, Shionogi, Shire, Up-To-Date, and Zafgen. J.M. received support and receipt of equipment, materials, drugs, medical writing, gifts or other services for current investigational study from Principia Biopharma (Sanofi). R. Baker reports research support/clinical trial funding for his institution from AbbVie, Acerta Pharma, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, CSL Behring, Daiichi Sankyo, Janssen-Cileg, Pfizer, Roche, Sanofi, Takeda, Technoclone, and Werfen. M.T. reports grants or contracts to the institution from Roche; consulting fees from Autolus, Caribou Biosciences, Genmab, Incyte, and Sobi; consulting fees and payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or education events from AbbVie, Amgen, BMS, Gilead Sciences, Janssen, MorphoSys, Novartis, Roche, and Takeda; and support for attending meetings and/or travel from AbbVie, BMS, Gilead Sciences, Janssen, Roche, and Takeda. P.Y.C. reports consultancy fees from Janssen Immunology, Nuvig, and Sobi, and speaker fees from Novartis. A.J.G.J. reports consultancy fees from Amgen and Novartis; speaker’s fees and travel cost payments from 3SBio, Amgen, and Novartis; international advisory board with Novartis; and research funding from Argenx, CSL Behring, Principia Biopharma (Sanofi), and Sobi. V.M. reports consultancy with Amgen, Argenx, Novartis, and Sobi; research funding from Grifols and Novartis; honoraria from Amgen, Argenx, Grifols, Novartis, and Sobi; and travel, accommodations, and expenses from Amgen. R. Bird reports being an investigator in clinical trials sponsored by Principia Biopharma (Sanofi) and Rigel, and serving on a speaker panel (nonremunerated) with Amgen. J.G. reports being an investigator in clinical trials sponsored by Principia Biopharma (Sanofi) and Rigel, and speaker fees from Amgen, Grifols, and Novartis. M.K. reports consultancy with Principia Biopharma Inc, a Sanofi company during the conduct of the study. T.G. reports research funding from Sobi; consulting for Cellphire and Sanofi; payment for lectures from Amgen and Sanofi; data safety and monitoring board for Palisade; honoraria from Sobi; and serving on advisory boards for Dova and Novartis. W.G. reports consultancy, honoraria, membership on an entity’s board of directors or advisory committees, travel and accommodations, and research funding from Alpine, Amgen, Argenx, Bayer, Grifols, Hutchmed, Kedrion, Novartis, Sanofi, Sobi, and UCB. A.D. reports current employment and is a current equity holder in publicly held company Sanofi. N.C. reports consultancy, honoraria, and research funding from Argenx, Grifols, Novartis, Principia, Rigel, Sanofi, Sobi, and UCB. The remaining authors declare no competing financial interests.
Figures
Similar articles
-
Rilzabrutinib for the Treatment of Immune Thrombocytopenia.Eur J Haematol. 2025 Jul;115(1):4-15. doi: 10.1111/ejh.14425. Epub 2025 Apr 13. Eur J Haematol. 2025. PMID: 40222822 Free PMC article. Review.
-
Efficacy and Safety Results With Rilzabrutinib, an Oral Bruton Tyrosine Kinase Inhibitor, in Patients With Immune Thrombocytopenia: Phase 2 Part B Study.Am J Hematol. 2025 Mar;100(3):439-449. doi: 10.1002/ajh.27539. Epub 2025 Jan 22. Am J Hematol. 2025. PMID: 39844469 Free PMC article. Clinical Trial.
-
Safety and efficacy of rilzabrutinib vs placebo in adults with immune thrombocytopenia: the phase 3 LUNA3 study.Blood. 2025 Jun 12;145(24):2914-2926. doi: 10.1182/blood.2024027336. Blood. 2025. PMID: 40090011 Clinical Trial.
-
Rilzabrutinib versus placebo in adults and adolescents with persistent or chronic immune thrombocytopenia: LUNA 3 phase III study.Ther Adv Hematol. 2023 Oct 18;14:20406207231205431. doi: 10.1177/20406207231205431. eCollection 2023. Ther Adv Hematol. 2023. PMID: 37869360 Free PMC article.
-
Romiplostim in chronic immune thrombocytopenic purpura.Clin Ther. 2009 Sep;31(9):1887-907. doi: 10.1016/j.clinthera.2009.09.013. Clin Ther. 2009. PMID: 19843480 Review.
Cited by
-
Efficacy and Safety of Syk and BTK Inhibitors in Immune Thrombocytopenia: A Comprehensive Review of Emerging Evidence.Mediators Inflamm. 2025 May 9;2025:5578929. doi: 10.1155/mi/5578929. eCollection 2025. Mediators Inflamm. 2025. PMID: 40385351 Free PMC article. Review.
-
Rilzabrutinib for the Treatment of Immune Thrombocytopenia.Eur J Haematol. 2025 Jul;115(1):4-15. doi: 10.1111/ejh.14425. Epub 2025 Apr 13. Eur J Haematol. 2025. PMID: 40222822 Free PMC article. Review.
-
Efficacy and Safety Results With Rilzabrutinib, an Oral Bruton Tyrosine Kinase Inhibitor, in Patients With Immune Thrombocytopenia: Phase 2 Part B Study.Am J Hematol. 2025 Mar;100(3):439-449. doi: 10.1002/ajh.27539. Epub 2025 Jan 22. Am J Hematol. 2025. PMID: 39844469 Free PMC article. Clinical Trial.
-
On the horizon: upcoming new agents for the management of ITP.Hematology Am Soc Hematol Educ Program. 2024 Dec 6;2024(1):692-699. doi: 10.1182/hematology.2024000596. Hematology Am Soc Hematol Educ Program. 2024. PMID: 39644072 Free PMC article. Review.
-
Design, Synthesis, and Evaluation of Trihalomethyl Ketone Derivatives of Neocarzilin A as Improved Antimetastatic Agents.ACS Bio Med Chem Au. 2024 Nov 5;4(6):331-341. doi: 10.1021/acsbiomedchemau.4c00087. eCollection 2024 Dec 18. ACS Bio Med Chem Au. 2024. PMID: 39712208 Free PMC article.
References
-
- Abrahamson PE, Hall SA, Feudjo-Tepie M, Mitrani-Gold FS, Logie J. The incidence of idiopathic thrombocytopenic purpura among adults: a population-based study and literature review. Eur J Haematol. 2009;83(2):83–89. - PubMed
-
- Feudjo-Tepie MA, Robinson NJ, Bennett D. Prevalence of diagnosed chronic immune thrombocytopenic purpura in the US: analysis of a large US claim database: a rebuttal. J Thromb Haemost. 2008;6(4):711–713. author reply 713. - PubMed
-
- Schoonen WM, Kucera G, Coalson J, et al. Epidemiology of immune thrombocytopenic purpura in the General Practice Research Database. Br J Haematol. 2009;145(2):235–244. - PubMed
-
- Segal JB, Powe NR. Prevalence of immune thrombocytopenia: analyses of administrative data. J Thromb Haemost. 2006;4(11):2377–2383. - PubMed
-
- Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85(3):174–180. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

