Cell-Surface ZnT8 Antibody Prevents and Reverses Autoimmune Diabetes in Mice
- PMID: 38387059
- PMCID: PMC11043063
- DOI: 10.2337/db23-0568
Cell-Surface ZnT8 Antibody Prevents and Reverses Autoimmune Diabetes in Mice
Abstract
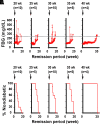
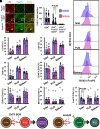
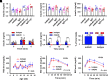
Type 1 diabetes (T1D) is an autoimmune disease in which pathogenic lymphocytes target autoantigens expressed in pancreatic islets, leading to the destruction of insulin-producing β-cells. Zinc transporter 8 (ZnT8) is a major autoantigen abundantly present on the β-cell surface. This unique molecular target offers the potential to shield β-cells against autoimmune attacks in T1D. Our previous work showed that a monoclonal antibody (mAb43) against cell-surface ZnT8 could home in on pancreatic islets and prevent autoantibodies from recognizing β-cells. This study demonstrates that mAb43 binds to exocytotic sites on the β-cell surface, masking the antigenic exposure of ZnT8 and insulin after glucose-stimulated insulin secretion. In vivo administration of mAb43 to NOD mice selectively increased the proportion of regulatory T cells in the islet, resulting in complete and sustained protection against T1D onset as well as reversal of new-onset diabetes. The mAb43-induced self-tolerance was reversible after treatment cessation, and no adverse effects were exhibited during long-term monitoring. Our findings suggest that mAb43 masking of the antigenic exposure of β-cells suppresses the immunological cascade from B-cell antigen presentation to T cell-mediated β-cell destruction, providing a novel islet-targeted and antigen-specific immunotherapy to prevent and reverse clinical T1D.
© 2024 by the American Diabetes Association.
Conflict of interest statement
Figures




References
-
- Vives M, Somoza N, Soldevila G, et al. . Reevaluation of autoantibodies to islet cell membrane in IDDM. Failure to detect islet cell surface antibodies using human islet cells as substrate. Diabetes 1992;41:1624–1631 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

