De novo variants in DENND5B cause a neurodevelopmental disorder
- PMID: 38387458
- PMCID: PMC10940048
- DOI: 10.1016/j.ajhg.2024.02.001
De novo variants in DENND5B cause a neurodevelopmental disorder
Abstract
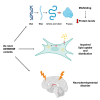
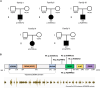
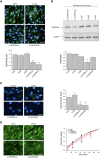
The Rab family of guanosine triphosphatases (GTPases) includes key regulators of intracellular transport and membrane trafficking targeting specific steps in exocytic, endocytic, and recycling pathways. DENND5B (Rab6-interacting Protein 1B-like protein, R6IP1B) is the longest isoform of DENND5, an evolutionarily conserved DENN domain-containing guanine nucleotide exchange factor (GEF) that is highly expressed in the brain. Through exome sequencing and international matchmaking platforms, we identified five de novo variants in DENND5B in a cohort of five unrelated individuals with neurodevelopmental phenotypes featuring cognitive impairment, dysmorphism, abnormal behavior, variable epilepsy, white matter abnormalities, and cortical gyration defects. We used biochemical assays and confocal microscopy to assess the impact of DENND5B variants on protein accumulation and distribution. Then, exploiting fluorescent lipid cargoes coupled to high-content imaging and analysis in living cells, we investigated whether DENND5B variants affected the dynamics of vesicle-mediated intracellular transport of specific cargoes. We further generated an in silico model to investigate the consequences of DENND5B variants on the DENND5B-RAB39A interaction. Biochemical analysis showed decreased protein levels of DENND5B mutants in various cell types. Functional investigation of DENND5B variants revealed defective intracellular vesicle trafficking, with significant impairment of lipid uptake and distribution. Although none of the variants affected the DENND5B-RAB39A interface, all were predicted to disrupt protein folding. Overall, our findings indicate that DENND5B variants perturb intracellular membrane trafficking pathways and cause a complex neurodevelopmental syndrome with variable epilepsy and white matter involvement.
Keywords: DENND5B; Rab GPTases; cell homeostasis; epilepsy; guanine nucleotide exchange factors; intellectual disability; lipid uptake and distribution; membrane trafficking; neurodevelopmental disorder.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.C. is an employee of GeneDx, LLC. The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic testing completed at Baylor Genetics Laboratories.
Figures



References
-
- Zerial M., McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

