Outcomes in solid organ transplant recipients with a pretransplant diagnosis of melanoma
- PMID: 38387619
- PMCID: PMC11144558
- DOI: 10.1016/j.ajt.2024.02.013
Outcomes in solid organ transplant recipients with a pretransplant diagnosis of melanoma
Abstract
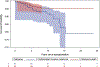
Melanoma causes significant morbidity in solid organ transplant recipients (SOTRs). Melanomas diagnosed before transplantation can recur with intensive immunosuppression, but outcomes have not been well studied. We evaluated 901 non-Hispanic White SOTRs with a pretransplant melanoma identified using linked transplant and cancer registry data in the United States. Most pretransplant melanomas were invasive (60.7%), and the median time from diagnosis to transplantation was 5.1 years. After transplantation, 41 SOTRs developed a new invasive melanoma, corresponding to 9-fold increased risk compared with the general population (standardized incidence ratio, 9.2; 95% confidence interval [CI], 6.6-12). Twenty-two SOTRs died from melanoma after transplantation, corresponding to 52-fold increased risk (standardized mortality ratio, 52; 95% CI, 33-79). Risk factors for posttransplant melanoma included age at transplantation (adjusted hazard ratio [HR], 2.86; 95% CI, 1.24-6.60; for age 55+ vs <55 years) and maintenance immunosuppression with cyclosporine/azathioprine (adjusted HR, 2.53; 95% CI, 1.08-5.90). Melanoma mortality was strongly elevated after a posttransplant melanoma diagnosis (HR, 35.6; 95% CI, 14.0-90.4; adjusted for cyclosporine/azathioprine maintenance therapy and calendar year of transplantation). In conclusion, SOTRs with a pretransplant melanoma are at risk of adverse melanoma-related outcomes after transplantation. These findings support thorough dermatologic evaluation prior to transplantation and frequent posttransplant surveillance.
Keywords: immunosuppression; melanoma; solid organ transplantation.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Figures
References
-
- Park CK, Dahlke EJ, Fung K, Kitchen J, Austin PC, Rochon PA, Chan AW. Melanoma incidence, stage, and survival after solid organ transplant: A population-based cohort study in Ontario, Canada. J Am Acad Dermatol. 2020;83(3):754–61. - PubMed
-
- Chapman JR, Sheil AG, Disney AP. Recurrence of cancer after renal transplantation. Transplant Proc. 2001;33(1–2):1830–1. - PubMed
-
- Penn I Malignant melanoma in organ allograft recipients. Transplantation. 1996;61(2):274–8. - PubMed
MeSH terms
Substances
Grants and funding
- 27307C0011/ES/NIEHS NIH HHS/United States
- HHSN261201000036C/CA/NCI NIH HHS/United States
- HHSN261201800013C/CA/NCI NIH HHS/United States
- HHSN261201000035I/CA/NCI NIH HHS/United States
- 75N92021D00009/HL/NHLBI NIH HHS/United States
- HHSN261201800012I/CA/NCI NIH HHS/United States
- HHSN261201800002B/CA/NCI NIH HHS/United States
- 75N98021D00006/OD/NIH HHS/United States
- HHSN261201000037C/CA/NCI NIH HHS/United States
- 27305C0011/ES/NIEHS NIH HHS/United States
- 75N96021D00006/ES/NIEHS NIH HHS/United States
- HHSN261201800004C/CA/NCI NIH HHS/United States
- N01 PC035143/CA/NCI NIH HHS/United States
- U58 DP003875/DP/NCCDPHP CDC HHS/United States
- 75N97021D00006/LM/NLM NIH HHS/United States
- HHSN261201800014C/CA/NCI NIH HHS/United States
- HHSN261201800016C/CA/NCI NIH HHS/United States
- N01 PC035139/CA/NCI NIH HHS/United States
- 75N99021D00009/OF/ORFDO NIH HHS/United States
- ZIA CP010150/ImNIH/Intramural NIH HHS/United States
- N01 PC035137/CA/NCI NIH HHS/United States
- U58 DP000807/DP/NCCDPHP CDC HHS/United States
- 27398C0011/ES/NIEHS NIH HHS/United States
- HHSN261201800016I/CA/NCI NIH HHS/United States
- U58 DP003933/DP/NCCDPHP CDC HHS/United States
- U58 DP000848/DP/NCCDPHP CDC HHS/United States
- HHSN261201800012C/CA/NCI NIH HHS/United States
- 75N90021D00009/CL/CLC NIH HHS/United States
- HHSN261201000035C/PC/NCI NIH HHS/United States
- HHSN261201000034C/CA/NCI NIH HHS/United States
- U58 DP003921/DP/NCCDPHP CDC HHS/United States
- 75N92021D00006/HL/NHLBI NIH HHS/United States
- HHSN261201800014I/CA/NCI NIH HHS/United States
- 75N96021D00009/ES/NIEHS NIH HHS/United States
- HHSN261201800002C/CA/NCI NIH HHS/United States
- HHSN261201800013I/CA/NCI NIH HHS/United States
- HHSN261201800006I/CA/NCI NIH HHS/United States
- U58 DP000824/DP/NCCDPHP CDC HHS/United States
LinkOut - more resources
Full Text Sources
Medical

