Orphan GPR52 as an emerging neurotherapeutic target
- PMID: 38387741
- PMCID: PMC11416161
- DOI: 10.1016/j.drudis.2024.103922
Orphan GPR52 as an emerging neurotherapeutic target
Abstract
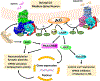
GPR52 is a highly conserved, brain-enriched, Gs/olf-coupled orphan G protein-coupled receptor (GPCR) that controls various cyclic AMP (cAMP)-dependent physiological and pathological processes. Stimulation of GPR52 activity might be beneficial for the treatment of schizophrenia, psychiatric disorders and other human neurological diseases, whereas inhibition of its activity might provide a potential therapeutic approach for Huntington's disease. Excitingly, HTL0048149 (HTL'149), an orally available GPR52 agonist, has been advanced into phase I human clinical trials for the treatment of schizophrenia. In this concise review, we summarize the current understanding of GPR52 receptor distribution as well as its structure and functions, highlighting the recent advances in drug discovery efforts towards small-molecule GPR52 ligands. The opportunities and challenges presented by targeting GPR52 for novel therapeutics are also briefly discussed.
Keywords: GPR52; Huntington’s disease; agonists; central nervous system disorders; drug discovery; orphan GPCR; schizophrenia.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
J.Z. and J.A.A., as well as their teams, were partially supported by MapLight Therapeutics, Inc. through an industry-funded collaborative research project, which was generally related to this manuscript.
Figures
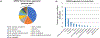


References
-
- Pierce KL et al. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. - PubMed
-
- Lee Y et al. Recent advances in structure-based drug design targeting class A G protein-coupled receptors utilizing crystal structures and computational simulations. J Med Chem. 2018;61:1–46. - PubMed
-
- Allen JA, Roth BL. Strategies to discover unexpected targets for drugs active at G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2011;51:117–144. - PubMed
-
- Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10:47–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

