The α-dystroglycan N-terminus is a broad-spectrum antiviral agent against SARS-CoV-2 and enveloped viruses
- PMID: 38387750
- PMCID: PMC7616797
- DOI: 10.1016/j.antiviral.2024.105837
The α-dystroglycan N-terminus is a broad-spectrum antiviral agent against SARS-CoV-2 and enveloped viruses
Abstract
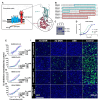
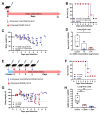
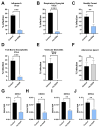
The COVID-19 pandemic has shown the need to develop effective therapeutics in preparedness for further epidemics of virus infections that pose a significant threat to human health. As a natural compound antiviral candidate, we focused on α-dystroglycan, a highly glycosylated basement membrane protein that links the extracellular matrix to the intracellular cytoskeleton. Here we show that the N-terminal fragment of α-dystroglycan (α-DGN), as produced in E. coli in the absence of post-translational modifications, blocks infection of SARS-CoV-2 in cell culture, human primary gut organoids and the lungs of transgenic mice expressing the human receptor angiotensin I-converting enzyme 2 (hACE2). Prophylactic and therapeutic administration of α-DGN reduced SARS-CoV-2 lung titres and protected the mice from respiratory symptoms and death. Recombinant α-DGN also blocked infection of a wide range of enveloped viruses including the four Dengue virus serotypes, influenza A virus, respiratory syncytial virus, tick-borne encephalitis virus, but not human adenovirus, a non-enveloped virus in vitro. This study establishes soluble recombinant α-DGN as a broad-band, natural compound candidate therapeutic against enveloped viruses.
Keywords: Broad-range antiviral; Coronaviruses; Enveloped viruses; Extracellular matrix; SARS-CoV-2; α-dystroglycan.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. Maria Giulia Bigotti, Katja Klein, Andrea Brancaccio and Yohei Yamauchi have patent #GB 2315095.6. issued to University of Bristol. Eng Eong Ooi has served in various advisory capacities on dengue vaccines for Sanofi Pasteur and MSD and served on the advisory board on dengue vaccines and antiviral drugs for Takeda. All other authors declare that they have no competing interests If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Banerjee I, Miyake Y, Nobs SP, Schneider C, Horvath P, Kopf M, Matthias P, Helenius A, Yamauchi Y. Influenza A virus uses the aggresome processing machinery for host cell entry. Science. 2014;346(6208):473–477. - PubMed
-
- Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

