Cardiovascular toxicities associated with bispecific T-cell engager therapy
- PMID: 38388168
- PMCID: PMC10882360
- DOI: 10.1136/jitc-2023-008518
Cardiovascular toxicities associated with bispecific T-cell engager therapy
Abstract
Background: Bispecific T-cell engagers (BTEs) are novel agents used to treat hematological malignancies. Early trials were underpowered to define cardiovascular adverse events (CVAE) and no large-scale studies systematically examined the CVAEs associated with BTEs.
Methods: Leveraging the US Food and Drug Administration's Adverse Event Reporting System-(FAERS), we identified the relative frequency of CVAEs after initiation of five BTE products approved by the Food and Drug Administration between 2014 and 2023 for the treatment of hematological malignancies. Adjusted reporting ORs (aROR) were used to identify disproportionate reporting of CVAEs with BTEs compared with background rates in the database. Fatality rates and risk ratios (RRs) for each adverse event (AE) were calculated.
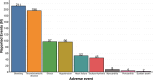
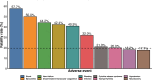
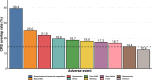
Results: From 3668 BTE-related cases reported to FAERS, 747 (20.4%) involved CVAEs. BTEs as a class were associated with fatal CVAEs (aROR 1.29 (95% CI 1.12 to 1.50)), an association mainly driven by teclistamab (aROR 2.44 (95% CI 1.65 to 3.60)). Teclistamab was also associated with a disproportionate risk of myocarditis (aROR 25.70 (95% CI 9.54 to 69.23)) and shock (aROR 3.63 (95% CI 2.30 to 5.74)), whereas blinatumomab was associated with a disproportionate risk of disseminated intravascular coagulation (aROR 3.02 (95% CI 1.98 to 4.60)) and hypotension (aROR 1.59 (95% CI 1.25 to 2.03)). CVAEs were more fatal compared with non-CVAEs (31.1% vs 17.4%; RR 1.76 (95% CI 1.54 to 2.03)). Most CVAEs (83.3%) did not overlap with cytokine release syndrome.
Conclusion: In the first postmarketing surveillance study of BTEs, CVAEs were involved in approximately one in five AE reports and carried a significant mortality risk.
Keywords: Antibodies, Bispecific; Cytotoxicity, Immunologic.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures


Comment in
-
Correspondence on 'Cardiovascular toxicities associated with bispecific T-cell engager therapy' by Sayed et al.J Immunother Cancer. 2024 Apr 22;12(4):e009137. doi: 10.1136/jitc-2024-009137. J Immunother Cancer. 2024. PMID: 38649281 Free PMC article. No abstract available.
References
-
- Goekbuget N, Dombret H, Bonifacio M, et al. . BLAST: A Confirmatory, single-arm, phase 2 study of Blinatumomab, a Bispecific T-cell Engager (bite®) antibody construct, in patients with minimal residual disease B-precursor acute Lymphoblastic leukemia (ALL). Blood 2014;124. 10.1182/blood.V124.21.379.379 - DOI
-
- Drugs@FDA [database on the Internet]. U.S. Food and Drug Administration . Drug approval package Blincyto (blinatumomab) injection, Available: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125557Orig1s000TO...
