Polyunsaturated fatty acids-induced ferroptosis suppresses pancreatic cancer growth
- PMID: 38388563
- PMCID: PMC10884029
- DOI: 10.1038/s41598-024-55050-4
Polyunsaturated fatty acids-induced ferroptosis suppresses pancreatic cancer growth
Abstract
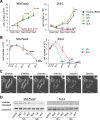
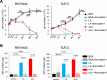
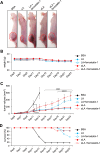
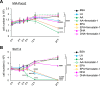
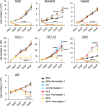
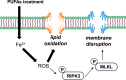
Despite recent advances in science and medical technology, pancreatic cancer remains associated with high mortality rates due to aggressive growth and no early clinical sign as well as the unique resistance to anti-cancer chemotherapy. Current numerous investigations have suggested that ferroptosis, which is a programed cell death driven by lipid oxidation, is an attractive therapeutic in different tumor types including pancreatic cancer. Here, we first demonstrated that linoleic acid (LA) and α-linolenic acid (αLA) induced cell death with necroptotic morphological change in MIA-Paca2 and Suit 2 cell lines. LA and αLA increased lipid peroxidation and phosphorylation of RIP3 and MLKL in pancreatic cancers, which were negated by ferroptosis inhibitor, ferrostatin-1, restoring back to BSA control levels. Similarly, intraperitoneal administration of LA and αLA suppresses the growth of subcutaneously transplanted Suit-2 cells and ameliorated the decreased survival rate of tumor bearing mice, while co-administration of ferrostatin-1 with LA and αLA negated the anti-cancer effect. We also demonstrated that LA and αLA partially showed ferroptotic effects on the gemcitabine-resistant-PK cells, although its effect was exerted late compared to treatment on normal-PK cells. In addition, the trial to validate the importance of double bonds in PUFAs in ferroptosis revealed that AA and EPA had a marked effect of ferroptosis on pancreatic cancer cells, but DHA showed mild suppression of cancer proliferation. Furthermore, treatment in other tumor cell lines revealed different sensitivity of PUFA-induced ferroptosis; e.g., EPA induced a ferroptotic effect on colorectal adenocarcinoma, but LA or αLA did not. Collectively, these data suggest that PUFAs can have a potential to exert an anti-cancer effect via ferroptosis in both normal and gemcitabine-resistant pancreatic cancer.
Keywords: Drug resistance; Ferroptosis; Linoleic acid; Pancreatic cancer; Poly unsaturated fatty acids; α-Linolenic acid.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

