α-lipoic acid modulates prostate cancer cell growth and bone cell differentiation
- PMID: 38388663
- PMCID: PMC10884017
- DOI: 10.1038/s41598-024-54479-x
α-lipoic acid modulates prostate cancer cell growth and bone cell differentiation
Abstract
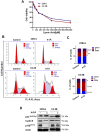
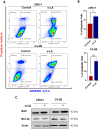
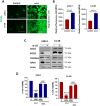
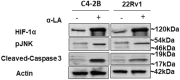
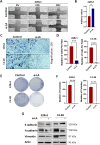
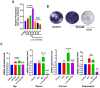
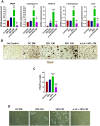
Prostate cancer (PCa) progression leads to bone modulation in approximately 70% of affected men. A nutraceutical, namely, α-lipoic acid (α-LA), is known for its potent anti-cancer properties towards various cancers and has been implicated in treating and promoting bone health. Our study aimed to explore the molecular mechanism behind the role of α-LA as therapeutics in preventing PCa and its associated bone modulation. Notably, α-LA treatment significantly reduced the cell viability, migration, and invasion of PCa cell lines in a dose-dependent manner. In addition, α-LA supplementation dramatically increased reactive oxygen species (ROS) levels and HIF-1α expression, which started the downstream molecular cascade and activated JNK/caspase-3 signaling pathway. Flow cytometry data revealed the arrest of the cell cycle in the S-phase, which has led to apoptosis of PCa cells. Furthermore, the results of ALP (Alkaline phosphatase) and TRAP (tartrate-resistant acid phosphatase) staining signifies that α-LA supplementation diminished the PCa-mediated differentiation of osteoblasts and osteoclasts, respectively, in the MC3T3-E1 and bone marrow macrophages (BMMs) cells. In summary, α-LA supplementation enhanced cellular apoptosis via increased ROS levels, HIF-1α expression, and JNK/caspase-3 signaling pathway in advanced human PCa cell lines. Also, the treatment of α-LA improved bone health by reducing PCa-mediated bone cell modulation.
Keywords: Bone modulation; HIF-1α; Osteoblasts; Osteoclasts; Prostate cancer; Reactive oxygen species (ROS); p-JNK; α-LA.
© 2024. The Author(s).
Conflict of interest statement
SKB is co-founder of Sanguine Diagnostics and Therapeutics, Inc. Other authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

