Mapping dysfunctional circuits in the frontal cortex using deep brain stimulation
- PMID: 38388734
- PMCID: PMC10917675
- DOI: 10.1038/s41593-024-01570-1
Mapping dysfunctional circuits in the frontal cortex using deep brain stimulation
Abstract
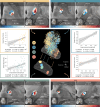
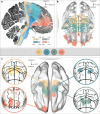
Frontal circuits play a critical role in motor, cognitive and affective processing, and their dysfunction may result in a variety of brain disorders. However, exactly which frontal domains mediate which (dys)functions remains largely elusive. We studied 534 deep brain stimulation electrodes implanted to treat four different brain disorders. By analyzing which connections were modulated for optimal therapeutic response across these disorders, we segregated the frontal cortex into circuits that had become dysfunctional in each of them. Dysfunctional circuits were topographically arranged from occipital to frontal, ranging from interconnections with sensorimotor cortices in dystonia, the primary motor cortex in Tourette's syndrome, the supplementary motor area in Parkinson's disease, to ventromedial prefrontal and anterior cingulate cortices in obsessive-compulsive disorder. Our findings highlight the integration of deep brain stimulation with brain connectomics as a powerful tool to explore couplings between brain structure and functional impairments in the human brain.
© 2024. The Author(s).
Conflict of interest statement
J.L.O. reports research grant support from Medtronic and Boston Scientific and is a consultant for Abbott, all DBS systems manufacturers, all outside of the submitted work. M.P. has received financial support for investigator-initiated trials from Boston Scientific, outside of the submitted work. M.M.R. reports grant support and honoraria for speaking from Medtronic and Boston Scientific, outside of the submitted work. J.V. reports grants and personal fees from Medtronic; grants and personal fees from Boston Scientific; and personal fees from Abbott, all outside of the submitted work. A.A.K. reports personal fees from Medtronic; personal fees from Boston Scientific; and personal fees from Stada Pharm, a pharmaceutical company, all outside of the submitted work. H.B. is a consultant for Alpha-Omega, a DBS systems manufacturer, outside of the submitted work. S.C. is a consultant for Medtronic and Boston Scientific, outside of the submitted work. A.H. is a consultant for FxNeuromodulation, a DBS-related startup company, and Abbott and reports lecture fees from Boston Scientific, all outside of the submitted work. B.H., I.A.S., N.R., S.O., K.B., C.N., P.R., P.Z., H.A., M.V., C.Z., B.S., P. Navratil, F.-C.Y., J.C.B., T.A.D., V.V.-V., E.J.L.A., P.R.F., P. Nanda, C.F., D.D.D., R.M.R., M.R.D., A.M., L.M.R., H.T., L.Z., E.M.J., P.A.S. and N.L. report no competing interests.
Figures





Update of
-
Mapping Dysfunctional Circuits in the Frontal Cortex Using Deep Brain Stimulation.medRxiv [Preprint]. 2023 Aug 25:2023.03.07.23286766. doi: 10.1101/2023.03.07.23286766. medRxiv. 2023. Update in: Nat Neurosci. 2024 Mar;27(3):573-586. doi: 10.1038/s41593-024-01570-1. PMID: 36945497 Free PMC article. Updated. Preprint.

