Phenotype-based targeted treatment of SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes
- PMID: 38388753
- PMCID: PMC10955037
- DOI: 10.1007/s00125-024-06099-3
Phenotype-based targeted treatment of SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes
Abstract
Aims/hypothesis: A precision medicine approach in type 2 diabetes could enhance targeting specific glucose-lowering therapies to individual patients most likely to benefit. We aimed to use the recently developed Bayesian causal forest (BCF) method to develop and validate an individualised treatment selection algorithm for two major type 2 diabetes drug classes, sodium-glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP1-RA).
Methods: We designed a predictive algorithm using BCF to estimate individual-level conditional average treatment effects for 12-month glycaemic outcome (HbA1c) between SGLT2i and GLP1-RA, based on routine clinical features of 46,394 people with type 2 diabetes in primary care in England (Clinical Practice Research Datalink; 27,319 for model development, 19,075 for hold-out validation), with additional external validation in 2252 people with type 2 diabetes from Scotland (SCI-Diabetes [Tayside & Fife]). Differences in glycaemic outcome with GLP1-RA by sex seen in clinical data were replicated in clinical trial data (HARMONY programme: liraglutide [n=389] and albiglutide [n=1682]). As secondary outcomes, we evaluated the impacts of targeting therapy based on glycaemic response on weight change, tolerability and longer-term risk of new-onset microvascular complications, macrovascular complications and adverse kidney events.
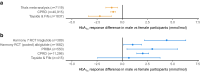
Results: Model development identified marked heterogeneity in glycaemic response, with 4787 (17.5%) of the development cohort having a predicted HbA1c benefit >3 mmol/mol (>0.3%) with SGLT2i over GLP1-RA and 5551 (20.3%) having a predicted HbA1c benefit >3 mmol/mol with GLP1-RA over SGLT2i. Calibration was good in hold-back validation, and external validation in an independent Scottish dataset identified clear differences in glycaemic outcomes between those predicted to benefit from each therapy. Sex, with women markedly more responsive to GLP1-RA, was identified as a major treatment effect modifier in both the UK observational datasets and in clinical trial data: HARMONY-7 liraglutide (GLP1-RA): 4.4 mmol/mol (95% credible interval [95% CrI] 2.2, 6.3) (0.4% [95% CrI 0.2, 0.6]) greater response in women than men. Targeting the two therapies based on predicted glycaemic response was also associated with improvements in short-term tolerability and long-term risk of new-onset microvascular complications.
Conclusions/interpretation: Precision medicine approaches can facilitate effective individualised treatment choice between SGLT2i and GLP1-RA therapies, and the use of routinely collected clinical features for treatment selection could support low-cost deployment in many countries.
Keywords: Bayesian non-parametric modelling; GLP1-receptor agonists; Heterogeneous treatment effects; Precision medicine; SGLT2-inhibitors; Type 2 diabetes.
© 2024. The Author(s).
Figures




References
-
- Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2022;65(12):1925–1966. doi: 10.1007/s00125-022-05787-2. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

