Age associated susceptibility to SARS-CoV-2 infection in the K18-hACE2 transgenic mouse model
- PMID: 38388916
- PMCID: PMC11009211
- DOI: 10.1007/s11357-024-01102-6
Age associated susceptibility to SARS-CoV-2 infection in the K18-hACE2 transgenic mouse model
Abstract
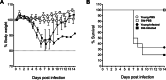
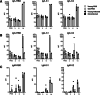
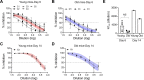
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is still an ongoing global health crisis. Clinical data indicate that the case fatality rate (CFR) is age dependent, with a higher CFR percentage in the elderly population. We compared the pathogenesis of SARS-CoV-2 in young and aged K18-hACE2 transgenic mice. We evaluated morbidity, mortality, viral titers, immune responses, and histopathology in SARS-CoV-2-infected young and old K18-hACE2 transgenic mice. Within the limitation of having a low number of mice per group, our results indicate that SARS-CoV-2 infection resulted in slightly higher morbidity, mortality, and viral replication in the lungs of old mice, which was associated with an impaired IgM response and altered cytokine and chemokine profiles. Results of this study increase our understanding of SARS-CoV-2 infectivity and immuno-pathogenesis in the elderly population.
Keywords: Aging; COVID-19; Cytokine storm; K18-hACE2 transgenic mouse; SARS-CoV-2 infection.
© 2024. The Author(s), under exclusive licence to American Aging Association.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
A human-ACE2 knock-in mouse model for SARS-CoV-2 infection recapitulates respiratory disorders but avoids neurological disease associated with the transgenic K18-hACE2 model.mBio. 2025 May 14;16(5):e0072025. doi: 10.1128/mbio.00720-25. Epub 2025 Apr 24. mBio. 2025. PMID: 40272151 Free PMC article.
-
The K18-Human ACE2 Transgenic Mouse Model Recapitulates Non-severe and Severe COVID-19 in Response to an Infectious Dose of the SARS-CoV-2 Virus.J Virol. 2022 Jan 12;96(1):e0096421. doi: 10.1128/JVI.00964-21. Epub 2021 Oct 20. J Virol. 2022. PMID: 34668775 Free PMC article.
-
Infectious Clones Produce SARS-CoV-2 That Causes Severe Pulmonary Disease in Infected K18-Human ACE2 Mice.mBio. 2021 Apr 20;12(2):e00819-21. doi: 10.1128/mBio.00819-21. mBio. 2021. PMID: 33879586 Free PMC article.
-
K18- and CAG-hACE2 Transgenic Mouse Models and SARS-CoV-2: Implications for Neurodegeneration Research.Molecules. 2022 Jun 28;27(13):4142. doi: 10.3390/molecules27134142. Molecules. 2022. PMID: 35807384 Free PMC article. Review.
-
SARS-CoV-2 immunity in animal models.Cell Mol Immunol. 2024 Feb;21(2):119-133. doi: 10.1038/s41423-023-01122-w. Epub 2024 Jan 18. Cell Mol Immunol. 2024. PMID: 38238440 Free PMC article. Review.
Cited by
-
Spatiotemporal Characterization of Changes in the Respiratory Tract and the Nervous System, Including the Eyes in SARS-CoV-2-Infected K18-hACE2 Mice.Viruses. 2025 Jul 9;17(7):963. doi: 10.3390/v17070963. Viruses. 2025. PMID: 40733579 Free PMC article.
References
-
- (WHO) WHO. WHO Coronavirus (COVID-19) Dashboard - https://covid19.who.int/ Geneva, Switzerland: WHO; 2023 .
-
- Flurkey K, Currer JM, Harrison DE. Mouse models in aging research. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL, editors. The Mouse in Biomedical Research. 2nd Ed. New York; Elsevier. 2007;3:637–72.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

