Vascular smooth muscle cell-specific Igf1r deficiency exacerbates the development of hypertension-induced cerebral microhemorrhages and gait defects
- PMID: 38388918
- PMCID: PMC11009188
- DOI: 10.1007/s11357-024-01090-7
Vascular smooth muscle cell-specific Igf1r deficiency exacerbates the development of hypertension-induced cerebral microhemorrhages and gait defects
Abstract
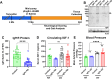
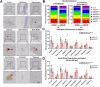
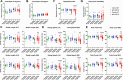
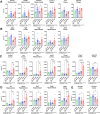
Cerebrovascular fragility and cerebral microhemorrhages (CMH) contribute to age-related cognitive impairment, mobility defects, and vascular cognitive impairment and dementia, impairing healthspan and reducing quality of life in the elderly. Insulin-like growth factor 1 (IGF-1) is a key vasoprotective growth factor that is reduced during aging. Circulating IGF-1 deficiency leads to the development of CMH and other signs of cerebrovascular dysfunction. Here our goal was to understand the contribution of IGF-1 signaling on vascular smooth muscle cells (VSMCs) to the development of CMH and associated gait defects. We used an inducible VSMC-specific promoter and an IGF-1 receptor (Igf1r) floxed mouse line (Myh11-CreERT2 Igf1rf/f) to knockdown Igf1r. Angiotensin II in combination with L-NAME-induced hypertension was used to elicit CMH. We observed that VSMC-specific Igf1r knockdown mice had accelerated development of CMH, and subsequent associated gait irregularities. These phenotypes were accompanied by upregulation of a cluster of pro-inflammatory genes associated with VSMC maladaptation. Collectively our findings support an essential role for VSMCs as a target for the vasoprotective effects of IGF-1, and suggest that VSMC dysfunction in aging may contribute to the development of CMH.
Keywords: Aging brain; Cerebral microhemorrhage; Cerebrovascular aging; Insulin-like growth factor 1; Vascular cognitive impairment; Vascular smooth muscle cells.
© 2024. The Author(s), under exclusive licence to American Aging Association.
Conflict of interest statement
Zoltan Ungvari is the Editor-in-Chief of Geroscience. Shannon Conley, Stefano Tarantini, Adam Nyul-Toth, and Andriy Yabluchanskiy are Associate Editors of Geroscience.
Figures


References
-
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 GM104938/GM/NIGMS NIH HHS/United States
- R03 AG070479/AG/NIA NIH HHS/United States
- R01 AG068295/AG/NIA NIH HHS/United States
- I01 BX005592/BX/BLRD VA/United States
- P30 AG050911/AG/NIA NIH HHS/United States
- K01 AG073614/AG/NIA NIH HHS/United States
- R01 EY019494/EY/NEI NIH HHS/United States
- T32 AG052363/AG/NIA NIH HHS/United States
- P30 EY021725/EY/NEI NIH HHS/United States
- P30 CA225520/CA/NCI NIH HHS/United States
- AHA941290/AHA/American Heart Association-American Stroke Association/United States
- P20 GM125528/GM/NIGMS NIH HHS/United States
- R01 AG070915/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

