Impairments of GABAergic transmission in hippocampus mediate increased susceptibility of epilepsy in the early stage of Alzheimer's disease
- PMID: 38388921
- PMCID: PMC10885444
- DOI: 10.1186/s12964-024-01528-7
Impairments of GABAergic transmission in hippocampus mediate increased susceptibility of epilepsy in the early stage of Alzheimer's disease
Abstract
Background: Patients with Alzheimer's disease (AD) are often co-morbid with unprovoked seizures, making clinical diagnosis and management difficult. Although it has an important role in both AD and epilepsy, abnormal γ-aminobutyric acid (GABA)ergic transmission is recognized only as a compensative change for glutamatergic damage. Neuregulin 1 (NRG1)-ErbB4 signaling can promote GABA release and suppress epileptogenesis, but its effects on cognition in AD are still controversial.
Methods: Four-month-old APPswe/PS1dE9 mice (APP mice) were used as animal models in the early stage of AD in this study. Acute/chronic chemical-kindling epilepsy models were established with pentylenetetrazol. Electroencephalogram and Racine scores were performed to assess seizures. Behavioral tests were used to assess cognition and emotion. Electrophysiology, western blot and immunofluorescence were performed to detect the alterations in synapses, GABAergic system components and NRG1-ErbB4 signaling. Furthermore, NRG1 was administrated intracerebroventricularly into APP mice and then its antiepileptic and cognitive effects were evaluated.
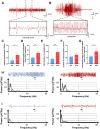
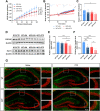
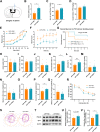
Results: APP mice had increased susceptibility to epilepsy and resulting hippocampal synaptic damage and cognitive impairment. Electrophysiological analysis revealed decreased GABAergic transmission in the hippocampus. This abnormal GABAergic transmission involved a reduction in the number of parvalbumin interneurons (PV+ Ins) and decreased levels of GABA synthesis and transport. We also found impaired NRG1-ErbB4 signaling which mediated by PV+ Ins loss. And NRG1 administration could effectively reduce seizures and improve cognition in four-month-old APP mice.
Conclusion: Our results indicated that abnormal GABAergic transmission mediated hippocampal hyperexcitability, further excitation/inhibition imbalance, and promoted epileptogenesis in the early stage of AD. Appropriate NRG1 administration could down-regulate seizure susceptibility and rescue cognitive function. Our study provided a potential direction for intervening in the co-morbidity of AD and epilepsy.
Keywords: Alzheimer's disease; Cognition; Epilepsy; ErbB4; GABAergic transmission; NRG1; Parvalbumin interneurons.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
ErbB4 deficiency exacerbates olfactory dysfunction in an early-stage Alzheimer's disease mouse model.Acta Pharmacol Sin. 2024 Dec;45(12):2497-2512. doi: 10.1038/s41401-024-01332-6. Epub 2024 Jul 9. Acta Pharmacol Sin. 2024. PMID: 38982150
-
ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation.Proc Natl Acad Sci U S A. 2010 Dec 14;107(50):21818-23. doi: 10.1073/pnas.1010669107. Epub 2010 Nov 24. Proc Natl Acad Sci U S A. 2010. PMID: 21106764 Free PMC article.
-
Neuregulin 1 Type I Overexpression Is Associated with Reduced NMDA Receptor-Mediated Synaptic Signaling in Hippocampal Interneurons Expressing PV or CCK.eNeuro. 2018 May 8;5(2):ENEURO.0418-17.2018. doi: 10.1523/ENEURO.0418-17.2018. eCollection 2018 Mar-Apr. eNeuro. 2018. PMID: 29740596 Free PMC article.
-
Seizures beget seizures: the quest for GABA as a key player.Crit Rev Neurobiol. 2006;18(1-2):135-44. doi: 10.1615/critrevneurobiol.v18.i1-2.140. Crit Rev Neurobiol. 2006. PMID: 17725516 Review.
-
Epileptic Mechanisms Shared by Alzheimer's Disease: Viewed via the Unique Lens of Genetic Epilepsy.Int J Mol Sci. 2021 Jul 1;22(13):7133. doi: 10.3390/ijms22137133. Int J Mol Sci. 2021. PMID: 34281185 Free PMC article. Review.
Cited by
-
Targeted activation of ErbB4 receptor ameliorates neuronal deficits and neuroinflammation in a food-borne polystyrene microplastic exposed mouse model.J Neuroinflammation. 2025 Mar 15;22(1):86. doi: 10.1186/s12974-025-03406-6. J Neuroinflammation. 2025. PMID: 40089796 Free PMC article.
-
Transcriptomic analyses of human brains with Alzheimer's disease identified dysregulated epilepsy-causing genes.Epilepsy Behav. 2025 Jul;168:110421. doi: 10.1016/j.yebeh.2025.110421. Epub 2025 Apr 17. Epilepsy Behav. 2025. PMID: 40250147
-
Transcriptomic analyses of human brains with Alzheimer's disease identified dysregulated epilepsy-causing genes.medRxiv [Preprint]. 2025 Jan 31:2025.01.02.25319900. doi: 10.1101/2025.01.02.25319900. medRxiv. 2025. Update in: Epilepsy Behav. 2025 Jul;168:110421. doi: 10.1016/j.yebeh.2025.110421. PMID: 39974070 Free PMC article. Updated. Preprint.
-
Synaptic and synchronic impairments in subcortical brain regions associated with early non-cognitive dysfunction in Alzheimer's disease.Neural Regen Res. 2026 Jan 1;21(1):248-264. doi: 10.4103/NRR.NRR-D-24-01052. Epub 2025 Jan 29. Neural Regen Res. 2026. PMID: 39885666 Free PMC article.
-
Glymphatic System Dysfunction in Elderly Patients with Late-Onset Epilepsy and Comorbid Chronic Insomnia Revealed by Diffusion Tensor Imaging Along the Perivascular Space (DTI-ALPS).Neuropsychiatr Dis Treat. 2025 Aug 4;21:1589-1598. doi: 10.2147/NDT.S510089. eCollection 2025. Neuropsychiatr Dis Treat. 2025. PMID: 40787617 Free PMC article.
References
-
- Li T-R, Han Y. China obotP-AAo: Insights on amyloid-related imaging abnormalities from the Pre-Alzheimer’s disease Alliance of China. AN 2022, 1.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

