Atorvastatin for reduction of 28-day mortality in severe and critical COVID-19 patients: a randomized controlled trial
- PMID: 38389078
- PMCID: PMC10885389
- DOI: 10.1186/s12931-024-02732-2
Atorvastatin for reduction of 28-day mortality in severe and critical COVID-19 patients: a randomized controlled trial
Abstract
Background: COVID-19 is an abnormal host response to the SARS-CoV-2 infection, which is associated with endothelial dysfunction and multi-organ failure. Atorvastatin has been proposed to reduce COVID-19 severity and mortality in chronic and de-novo users.
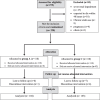
Methods: This randomized double-blind trial included 220 COVID-19 patients admitted to Mansoura University's isolation hospital in Egypt. One hundred and ten cases were given 40 mg of atorvastatin once daily for 28 days (group A), while 110 received a placebo (group B). All patients received treatment as per hospital protocol. The primary outcome is all-cause mortality at 28 days. We also tracked 6-month mortality, time to clinical improvement, the risk of invasive mechanical ventilation, acute kidney injury, potential adverse events, and hospital and intensive care length of stay.
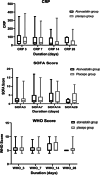
Results: The 28-day all-cause mortality was 52/104 (50%) in group A vs. 54/103 (52.4%) in group B, odds ratio (OR) = 0.907 (0.526, 1.565), P = 0.727; adjusted OR = 0.773 (0.407, 1.47), P = 0.433. Six-month mortality occurred in 53/102 (52%) and 59/79 (60.8%) in group A vs. B, respectively, P = 0.208. Among hospital survivors in group A vs. group B, the median time to clinical improvement was 10 days (7-14) vs. 10 (7-15), P = 0.715; the duration of hospital stay was 10 days (7-14) vs. 10 (8-17), P = 0.378. Discontinuation was higher in group B (four vs. one), but statistically insignificant, P = 0.369.
Conclusions: In adults with severe or critical COVID-19, atorvastatin did not reduce the risk of 28-day or 6-month mortality and did not shorten the length of hospital stay or time to clinical improvement. Trial registration Clinical Trial Registry (NCT04952350) on July 1st, 2021. https://clinicaltrials.gov/ct2/show/NCT04952350.
Keywords: Atorvastatin; COVID-19; Coronavirus; Mortality; SARS-CoV-2; Statins, hmg coa.
© 2024. The Author(s).
Conflict of interest statement
All authors report no financial or other competing interests.
Figures
Similar articles
-
Atorvastatin for reduction of 28-day mortality in hospitalized COVID-19 patients: study protocol for a randomized, double-blinded, placebo-controlled, clinical trial.Trials. 2022 Aug 8;23(1):636. doi: 10.1186/s13063-022-06619-9. Trials. 2022. PMID: 35941669 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Atorvastatin and Aspirin as Adjuvant Therapy in Patients with SARS-CoV-2 Infection: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 30;21(1):902. doi: 10.1186/s13063-020-04840-y. Trials. 2020. PMID: 33126910 Free PMC article.
-
A randomized open-label trial to evaluate the efficacy and safety of triple therapy with aspirin, atorvastatin, and nicorandil in hospitalised patients with SARS Cov-2 infection: A structured summary of a study protocol for a randomized controlled trial.Trials. 2021 Jul 15;22(1):451. doi: 10.1186/s13063-021-05361-y. Trials. 2021. PMID: 34266452 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review.Cochrane Database Syst Rev. 2020 May 14;5(5):CD013600. doi: 10.1002/14651858.CD013600. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Jul 10;7:CD013600. doi: 10.1002/14651858.CD013600.pub2. PMID: 32406927 Free PMC article. Updated.
Cited by
-
Characteristics of symptoms and establishment of a predictive model for PICS in mechanically ventilated patients with severe pneumonia: a retrospective study.Eur J Med Res. 2025 Apr 10;30(1):264. doi: 10.1186/s40001-025-02547-x. Eur J Med Res. 2025. PMID: 40211419 Free PMC article.
-
Effect of IL-6R blockade on plasma lipids and clinical outcomes among hospitalized patients with COVID-19 infection.J Lipid Res. 2024 Jun;65(6):100568. doi: 10.1016/j.jlr.2024.100568. Epub 2024 May 23. J Lipid Res. 2024. PMID: 38795859 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous