Hypoxic glioblastoma-cell-derived extracellular vesicles impair cGAS-STING activity in macrophages
- PMID: 38389103
- PMCID: PMC10882937
- DOI: 10.1186/s12964-024-01523-y
Hypoxic glioblastoma-cell-derived extracellular vesicles impair cGAS-STING activity in macrophages
Abstract
Background: Solid tumors such as glioblastoma (GBM) exhibit hypoxic zones that are associated with poor prognosis and immunosuppression through multiple cell intrinsic mechanisms. However, release of extracellular vesicles (EVs) has the potential to transmit molecular cargos between cells. If hypoxic cancer cells use EVs to suppress functions of macrophages under adequate oxygenation, this could be an important underlying mechanism contributing to the immunosuppressive and immunologically cold tumor microenvironment of tumors such as GBM.
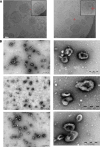
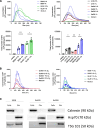
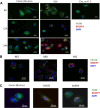
Methods: EVs were isolated by differential ultracentrifugation from GBM cell culture supernatant. EVs were thoroughly characterized by transmission and cryo-electron microscopy, nanoparticle tracking analysis (NTA), and EV marker expression by Western blot and fluorescent NTA. EV uptake by macrophage cells was observed using confocal microscopy. The transfer of miR-25/93 as an EV cargo to macrophages was confirmed by miRNA real-time qPCR. The impact of miR-25/93 on the polarization of recipient macrophages was shown by transcriptional analysis, cytokine secretion and functional assays using co-cultured T cells.
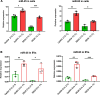
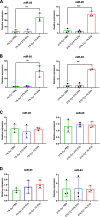
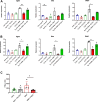
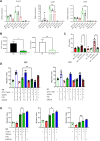
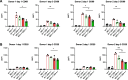
Results: We show that indirect effects of hypoxia can have immunosuppressive consequences through an EV and microRNA dependent mechanism active in both murine and human tumor and immune cells. Hypoxia enhanced EV release from GBM cells and upregulated expression of miR-25/93 both in cells and in EV cargos. Hypoxic GBM-derived EVs were taken up by macrophages and the miR-25/93 cargo was transferred, leading to impaired cGAS-STING pathway activation revealed by reduced type I IFN expression and secretion by macrophages. The EV-treated macrophages downregulated expression of M1 polarization-associated genes Cxcl9, Cxcl10 and Il12b, and had reduced capacity to attract activated T cells and to reactivate them to release IFN-γ, key components of an efficacious anti-tumor immune response.
Conclusions: Our findings suggest a mechanism by which immunosuppressive consequences of hypoxia mediated via miRNA-25/93 can be exported from hypoxic GBM cells to normoxic macrophages via EVs, thereby contributing to more widespread T-cell mediated immunosuppression in the tumor microenvironment.
Keywords: Extracellular vesicles; Glioblastoma; Hypoxia/ miRNA; cGAS-STING pathway.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

