Dapagliflozin in chronic kidney disease: cost-effectiveness beyond the DAPA-CKD trial
- PMID: 38389710
- PMCID: PMC10883141
- DOI: 10.1093/ckj/sfae025
Dapagliflozin in chronic kidney disease: cost-effectiveness beyond the DAPA-CKD trial
Abstract
Background: The Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) trial enrolled patients with estimated glomerular filtration rate 25-75 mL/min/1.73 m2 and urine albumin-to-creatinine ratio >200 mg/g. The Dapagliflozin Effect on CardiovascuLAR Events-Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial enrolled patients with type 2 diabetes, a higher range of kidney function and no albuminuria criterion. The study objective was to estimate the cost-effectiveness of dapagliflozin in a broad chronic kidney disease population based on these two trials in the UK, Spain, Italy and Japan.
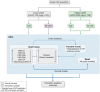
Methods: We adapted a published Markov model based on the DAPA-CKD trial but to a broader population, irrespective of urine albumin-to-creatinine ratio, using patient-level data from the DAPA-CKD and DECLARE-TIMI 58 trials. We sourced cost and utility inputs from literature and the DAPA-CKD trial. The analysis considered healthcare system perspectives over a lifetime horizon.
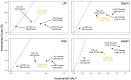
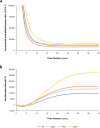
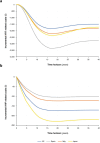
Results: Treatment with dapagliflozin was predicted to attenuate disease progression and extend projected life expectancy by 0.64 years (12.5 versus 11.9 years, undiscounted) in the UK, with similar estimates in other settings. Clinical benefits translated to mean quality-adjusted life year (QALY; discounted) gains between 0.45 and 0.68 years across countries. Incremental cost-effectiveness ratios in the UK, Spain, Italy and Japan ($10 676/QALY, $14 479/QALY, $7771/QALY and $13 723/QALY, respectively) were cost-effective at country-specific willingness-to-pay thresholds. Subgroup analyses suggest dapagliflozin is cost-effective irrespective of urinary albumin-to-creatine ratio and type 2 diabetes status.
Conclusion: Treatment with dapagliflozin may be cost-effective for patients across a wider spectrum of estimated glomerular filtration rates and albuminuria than previously demonstrated, with or without type 2 diabetes, in the UK, Spanish, Italian and Japanese healthcare systems.
Keywords: SGLT2 inhibitor; albuminuria; chronic kidney disease; cost-effectiveness; dapagliflozin.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
P.M., J.A.D. and P.D.G. are employees of Health Economics and Outcomes Research Ltd, Cardiff, UK. Health Economics and Outcomes Research Ltd received fees from AstraZeneca in relation to this study. D.C.W. provided ongoing consultancy services to AstraZeneca in the last 2 years and has received honoraria and/or consultancy fees from Amgen, AstraZeneca, Boehringer Ingelheim, Bayer, Eledon, Galderma, Gilead, GlaxoSmithKline, George Clinical, Janssen, Merck Sharp and Dohme, ProKidney, Takeda, Vifor and Zydus. He also reports speaking fees from Astellas, AstraZeneca and Vifor, and support for travel/meeting attendance from Astellas, AstraZeneca and Pro. He has served on DSMBs for Eledon, Galderma, Merck, ProKidney and Pathalys. He is a member of the International Society of Nephrology and National Institute of Health Research, UK. P.R. has received honoraria to Steno Diabetes Center Copenhagen for consultancy from AstraZeneca, Astellas, Bayer, Boehringer Ingelheim, Gilead, Novo Nordisk, Merck, Mundipharma, Sanofi and Vifor, and research support from AstraZeneca, Bayer and Novo Nordisk. G.M.C. received fees from AstraZeneca for service on the DAPA-CKD trial steering committee. He serves on the Board of Directors for Satellite Healthcare, a non-profit dialysis provider. He has received research grants to his institution from NIDDK, NIAID and CSL Behring. He has served on trial-steering committees with Akebia, AstraZeneca, Gilead, Sanifit and Vertex. He has served as an advisor to Applaud, Ardelyx, CloudCath, Durect, Eliaz Therapeutics, Miromatrix, Outset, Renibus, Unicycive and Vertex. He has served on DSMBs for NIDDK, Bayer, Mineralys and ReCor. He also declares stock or stock options for Applaud, Ardelyx, CloudCath, Durect, Eliaz Therapeutics, Miromatrix, Outset and Renibus. R.C.-R. has received honoraria as consultant from AstraZeneca, Boehringer Ingelheim, Bayer, Chinook, AbbVie and Novo Nordisk, and research support from AstraZeneca, Boehringer Ingelheim, Roche and Novo Nordisk. He has received speaking fees from AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Amgen. K.T. has honoraria as lecture fee from Novartis, AstraZeneca, Ono, Daiichi-Sankyo, Takeda, Otsuka, Bayer and Kyowa-Kirin. He has received research support from AstraZeneca, Ono, Bayer, Kyowa-Kirin, Otsuka, Takeda and Daiichi-Sankyo. S.B. and J.J.G.S. are employees of AstraZeneca.
Figures

Similar articles
-
Cost-Effectiveness of Dapagliflozin as a Treatment for Chronic Kidney Disease: A Health-Economic Analysis of DAPA-CKD.Clin J Am Soc Nephrol. 2022 Dec;17(12):1730-1741. doi: 10.2215/CJN.03790322. Epub 2022 Nov 2. Clin J Am Soc Nephrol. 2022. PMID: 36323444 Free PMC article.
-
Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial.Lancet Diabetes Endocrinol. 2021 Nov;9(11):755-766. doi: 10.1016/S2213-8587(21)00243-6. Epub 2021 Oct 4. Lancet Diabetes Endocrinol. 2021. PMID: 34619106 Clinical Trial.
-
The long-term effects of dapagliflozin in chronic kidney disease: a time-to-event analysis.Nephrol Dial Transplant. 2024 Nov 27;39(12):2040-2047. doi: 10.1093/ndt/gfae106. Nephrol Dial Transplant. 2024. PMID: 38730538 Free PMC article. Clinical Trial.
-
Translating the efficacy of dapagliflozin in chronic kidney disease to lower healthcare resource utilization and costs: a medical care cost offset analysis.J Med Econ. 2023 Jan-Dec;26(1):1407-1416. doi: 10.1080/13696998.2023.2264715. Epub 2023 Oct 31. J Med Econ. 2023. PMID: 37807895 Review.
-
The Role of Dapagliflozin in the Management of Heart Failure: An Update on the Emerging Evidence.Ther Clin Risk Manag. 2021 Aug 12;17:823-830. doi: 10.2147/TCRM.S275076. eCollection 2021. Ther Clin Risk Manag. 2021. PMID: 34408424 Free PMC article. Review.
Cited by
-
Economic evaluation of adding dapagliflozin to standard care in the treatment of chronic kidney disease: a systematic review.BMC Nephrol. 2024 Dec 18;25(1):465. doi: 10.1186/s12882-024-03901-7. BMC Nephrol. 2024. PMID: 39695416 Free PMC article.
-
Practical Considerations and Implementation of Sodium-Glucose Co-Transporter-2 Inhibitors in Chronic Kidney Disease: Who, When, and How? A Position Statement by Nephrologists.J Prim Care Community Health. 2024 Jan-Dec;15:21501319241259905. doi: 10.1177/21501319241259905. J Prim Care Community Health. 2024. PMID: 39143759 Free PMC article.
-
Cost-Effectiveness of Adding Dapagliflozin and Empagliflozin to Standard Treatment for Diabetic Kidney Disease in China.Clin Drug Investig. 2025 Jul 14. doi: 10.1007/s40261-025-01462-7. Online ahead of print. Clin Drug Investig. 2025. PMID: 40658333
-
Cost-Effectiveness Analysis of SGLT2 Inhibitors for Cardio-Renal-Metabolic Disease Based on Data from Japanese Studies.Adv Ther. 2025 Jun;42(6):2888-2905. doi: 10.1007/s12325-025-03157-z. Epub 2025 Apr 29. Adv Ther. 2025. PMID: 40299278 Free PMC article.
-
Pharmacological Nephroprotection in Chronic Kidney Disease Patients with Type 2 Diabetes Mellitus-Clinical Practice Position Statement of the Polish Society of Nephrology.Int J Mol Sci. 2024 Dec 2;25(23):12941. doi: 10.3390/ijms252312941. Int J Mol Sci. 2024. PMID: 39684653 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

