Development of CD46 targeted alpha theranostics in prostate cancer using 134Ce/225Ac-Macropa-PEG4-YS5
- PMID: 38389832
- PMCID: PMC10879874
- DOI: 10.7150/thno.92742
Development of CD46 targeted alpha theranostics in prostate cancer using 134Ce/225Ac-Macropa-PEG4-YS5
Abstract
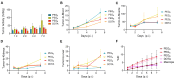
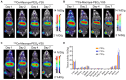
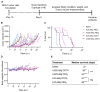
Rationale: 225Ac, a long-lived α-emitter with a half-life of 9.92 days, has garnered significant attention as a therapeutic radionuclide when coupled with monoclonal antibodies and other targeting vectors. Nevertheless, its clinical utility has been hampered by potential off-target toxicity, a lack of optimized chelators for 225Ac, and limitations in radiolabeling methods. In a prior study evaluating the effectiveness of CD46-targeted radioimmunotherapy, we found great therapeutic efficacy but also significant toxicity at higher doses. To address these challenges, we have developed a radioimmunoconjugate called 225Ac-Macropa-PEG4-YS5, incorporating a stable PEGylated linker to maximize tumoral uptake and increase tumor-to-background ratios. Our research demonstrates that this conjugate exhibits greater anti-tumor efficacy while minimizing toxicity in prostate cancer 22Rv1 tumors. Methods: We synthesized Macropa.NCS and Macropa-PEG4/8-TFP esters and prepared Macropa-PEG0/4/8-YS5 (with nearly ~1:1 ratio of macropa chelator to antibody YS5) as well as DOTA-YS5 conjugates. These conjugates were then radiolabeled with 225Ac in a 2 M NH4OAc solution at 30 °C, followed by purification using YM30K centrifugal purification. Subsequently, we conducted biodistribution studies and evaluated antitumor activity in nude mice (nu/nu) bearing prostate 22Rv1 xenografts in both single-dose and fractionated dosing studies. Micro-PET imaging studies were performed with 134Ce-Macropa-PEG0/4/8-YS5 in 22Rv1 xenografts for 7 days. Toxicity studies were also performed in healthy athymic nude mice. Results: As expected, we achieved a >95% radiochemical yield when labeling Macropa-PEG0/4/8-YS5 with 225Ac, regardless of the chelator ratios (ranging from 1 to 7.76 per YS5 antibody). The isolated yield exceeded 60% after purification. Such high conversions were not observed with the DOTA-YS5 conjugate, even at a higher ratio of 8.5 chelators per antibody (RCY of 83%, an isolated yield of 40%). Biodistribution analysis at 7 days post-injection revealed higher tumor uptake for the 225Ac-Macropa-PEG4-YS5 (82.82 ± 38.27 %ID/g) compared to other conjugates, namely 225Ac-Macropa-PEG0/8-YS5 (38.2 ± 14.4/36.39 ± 12.4 %ID/g) and 225Ac-DOTA-YS5 (29.35 ± 7.76 %ID/g). The PET Imaging of 134Ce-Macropa-PEG0/4/8-YS5 conjugates resulted in a high tumor uptake, and tumor to background ratios. In terms of antitumor activity, 225Ac-Macropa-PEG4-YS5 exhibited a substantial response, leading to prolonged survival compared to 225Ac-DOTA-YS5, particularly when administered at 4.625 kBq doses, in single or fractionated dose regimens. Chronic toxicity studies observed mild to moderate renal toxicity at 4.625 and 9.25 kBq doses. Conclusions: Our study highlights the promise of 225Ac-Macropa-PEG4-YS5 for targeted alpha particle therapy. The 225Ac-Macropa-PEG4-YS5 conjugate demonstrates improved biodistribution, reduced off-target binding, and enhanced therapeutic efficacy, particularly at lower doses, compared to 225Ac-DOTA-YS5. Incorporating theranostic 134Ce PET imaging further enhances the versatility of macropa-PEG conjugates, offering a more effective and safer approach to cancer treatment. Overall, this methodology has a high potential for broader clinical applications.
Keywords: PEG linkers; YS5 antibody; actinium-225; cerium-134; macropa; targeted alpha therapy; theranostics.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Asadian S, Mirzaei H, Kalantari BA, Davarpanah MR, Mohamadi M, Shpichka A. et al. β-radiating radionuclides in cancer treatment, novel insight into promising approach. Pharmacol Res. 2020;160:105070. - PubMed
-
- Scholl C, Bundschuh RA, Hirzebruch S, Glanert T, Wei X, Kürpig S. et al. Radionuclide intake risks in the clinical administration of 223RaCl2. J Radiol Prot. 2019;39:387–98. - PubMed
-
- Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Eng J Med. 2013;369:213–23. - PubMed

