Design, synthesis and biological evaluation of novel morpholinopyrimidine-5-carbonitrile derivatives as dual PI3K/mTOR inhibitors
- PMID: 38389871
- PMCID: PMC10880895
- DOI: 10.1039/d3md00693j
Design, synthesis and biological evaluation of novel morpholinopyrimidine-5-carbonitrile derivatives as dual PI3K/mTOR inhibitors
Abstract
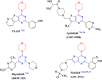
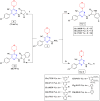
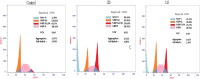
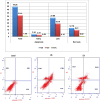
In this study, novel morpholinopyrimidine-5-carbonitriles were designed and synthesized as dual PI3K/mTOR inhibitors and apoptosis inducers. The integration of a heterocycle at position 2, with or without spacers, of the new key intermediate 2-hydrazinyl-6-morpholinopyrimidine-5-carbonitrile (5) yielded compounds 6-10, 11a-c and 12a-h. The National Cancer Institute (USA) tested all compounds for antiproliferative activity. Schiff bases, 12a-h analogs, were the most active ones. The most promising compounds 12b and 12d exhibited excellent antitumor activity against the leukemia SR cell line, which is the most sensitive cell line, with IC50 0.10 ± 0.01 and 0.09 ± 0.01 μM, respectively, along with significant effects on PI3Kα/PI3Kβ/PI3Kδ with IC50 values of 0.17 ± 0.01, 0.13 ± 0.01 and 0.76 ± 0.04 μM, respectively, for 12b and 1.27 ± 0.07, 3.20 ± 0.16 and 1.98 ± 0.11, respectively, for 12d compared to LY294002. Compared to Afinitor, these compounds inhibited mTOR with IC50 values of 0.83 ± 0.05 and 2.85 ± 0.17 μM, respectively. Annexin-V and propidium iodide (PI) double labeling showed that compounds 12b and 12d promote cytotoxic leukemia SR apoptosis. Compounds 12b and 12d also caused a G2/M cell cycle arrest in the leukaemia SR cell line. The findings of this study indicate that the highest effect was observed for 12b, which was supported by western blot and docking analysis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Akhtar W. Nainwal L. M. Khana M. F. Verma G. Chashoo G. Bakht A. Iqbal M. Akhtar M. Shaquiquzzaman M. Alam M. M. Synthesis, COX-2 inhibition and metabolic stability studies of 6- (4-fluorophenyl)-pyrimidine-5-carbonitrile derivatives as anticancer and anti-inflammatory agents. J. Fluorine Chem. 2020;236:109579. doi: 10.1016/j.jfluchem.2020.109579. doi: 10.1016/j.jfluchem.2020.109579. - DOI
-
- Saini A. Kumar M. Bhatt S. Saini V. Malik A. Cancer causes and treatments. Int. J. Pharm. Sci. Res. 2020;11:3109–3122. doi: 10.13040/IJPSR.0975-8232.11(7).3109-22. - DOI
LinkOut - more resources
Full Text Sources
Chemical Information
Research Materials
Miscellaneous

