In silico analysis of luteolin derivatives as antibacterial agents targeting DNA gyrase and CTX-M-15 extended-spectrum β-lactamase of Escherichia coli
- PMID: 38389968
- PMCID: PMC10880919
- DOI: 10.4103/JAPTR.JAPTR_217_23
In silico analysis of luteolin derivatives as antibacterial agents targeting DNA gyrase and CTX-M-15 extended-spectrum β-lactamase of Escherichia coli
Abstract
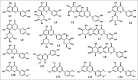
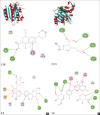
Luteolin exhibited antibacterial activity against Escherichia coli and its chemical structure similar to that of ciprofloxacin (CPF) which works by inhibiting DNA gyrase. Filtrate from passion fruit extract containing luteolin and its derivatives could inhibit extended-spectrum β-lactamase (ESBL)-producing E. coli. Antibacterial compounds that can also inhibit ESBL will be valuable compounds to overcome the problem of resistant bacteria. This study aimed to ensure the potency of luteolin and luteolin derivatives targeting DNA gyrase and ESBL by in silico approach. Docking simulation of ligands L1-L14 was performed using AutoDock Vina, and pharmacokinetics and toxicity (absorption, distribution, metabolism, excretion, and toxicity) profiles were predicted by pKCSM online. The docking result revealed higher binding affinity on DNA gyrase (PDB.1KZN) of 12 luteolin derivatives (energy <-7.6 kcal/mol) compared to CPF and higher affinity (energy <-6.27 kcal/mol) of all compounds than clavulanic acid against ESBL CTX-M-15 (PDB.4HBU). The compounds could be absorbed through the human intestine moderately, which showed low permeability to blood-brain barrier, nontoxic and nonhepatotoxic. The most active luteolin glycoside (L6) is capable to inhibit DNA gyrase and ESBL from E. coli which provided the potential against resistant bacteria and was promoted as lead compounds to be developed further.
Keywords: Absorption; DNA gyrase; and toxicity; distribution; docking; excretion; extended-spectrum β-lactamase CTX-M-15; luteolin derivatives; metabolism.
Copyright: © 2024 Journal of Advanced Pharmaceutical Technology & Research.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
An In Vitro and In Silico Study of Luteolin-Loaded Zinc Oxide Nanoparticles: Enhancing Bioactivity and Efficacy for Advanced Therapeutic Applications Against Cariogenic Microorganisms.Cureus. 2024 Aug 28;16(8):e68058. doi: 10.7759/cureus.68058. eCollection 2024 Aug. Cureus. 2024. PMID: 39347219 Free PMC article.
-
[Investigation of beta-lactamase genes and clonal relationship among the extended-spectrum beta-lactamase producing nosocomial Escherichia coli isolates].Mikrobiyol Bul. 2015 Jan;49(1):15-25. doi: 10.5578/mb.8437. Mikrobiyol Bul. 2015. PMID: 25706727 Turkish.
-
Efficacy of Andrographis paniculata against extended spectrum β-lactamase (ESBL) producing E. coli.BMC Complement Altern Med. 2018 Sep 3;18(1):244. doi: 10.1186/s12906-018-2312-8. BMC Complement Altern Med. 2018. PMID: 30176904 Free PMC article.
-
Emergence and Comparative Genomics Analysis of Extended-Spectrum-β-Lactamase-Producing Escherichia coli Carrying mcr-1 in Fennec Fox Imported from Sudan to China.mSphere. 2019 Nov 20;4(6):e00732-19. doi: 10.1128/mSphere.00732-19. mSphere. 2019. PMID: 31748247 Free PMC article.
-
Prevalence of extended-spectrum β-lactamase-producing Escherichia coli and residual antimicrobials in the environment in Vietnam.Anim Health Res Rev. 2017 Dec;18(2):128-135. doi: 10.1017/S1466252317000160. Anim Health Res Rev. 2017. PMID: 29665885 Review.
Cited by
-
A Stroll Through Saffron Fields, Cannabis Leaves, and Cherry Reveals the Path to Waste-Derived Antimicrobial Bioproducts.Pharmaceuticals (Basel). 2025 Jul 3;18(7):1003. doi: 10.3390/ph18071003. Pharmaceuticals (Basel). 2025. PMID: 40732292 Free PMC article.
-
PknB and STP as potential targets of luteolin in combating Trueperella pyogenes infections.Sci Rep. 2025 Jul 3;15(1):23830. doi: 10.1038/s41598-025-08698-5. Sci Rep. 2025. PMID: 40610526 Free PMC article.
References
-
- Kawamoto Y, Kosai K, Yamakawa H, Kaku N, Uno N, Morinaga Y, et al. Detection of extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae using the MALDI biotyper selective testing of antibiotic resistance-β-lactamase (MBT STAR-BL) assay. J Microbiol Methods. 2019;160:154–6. - PubMed
-
- Raini M. Antibiotik Golongan Fluorokuinolon: Manfaat Dan Kerugian (Fluoroquinolones Antibiotics: Benefit and Side Effects) Media Litbangkes. 2016;26:163–74.
LinkOut - more resources
Full Text Sources

