Reassessing the efficacy of bevacizumab in newly diagnosed glioblastoma: A systematic review and external pseudodata-based analysis
- PMID: 38390032
- PMCID: PMC10883711
- DOI: 10.1093/noajnl/vdad174
Reassessing the efficacy of bevacizumab in newly diagnosed glioblastoma: A systematic review and external pseudodata-based analysis
Abstract
Background: First-line use of bevacizumab for glioblastoma (GBM) was evaluated in 2 phase 3 randomized controlled trials (RCT), demonstrating an impact on progression-free survival but not overall survival (OS). However, the crossover events of these trials raised concerns regarding the reliability of this latter analysis. In this study, we conducted an external control-based reassessment of the bevacizumab efficacy in newly diagnosed GBM (ndGBM) against the standard Stupp protocol.
Methods: A systematic review of the literature was conducted to identify the phase 3 RCTs in ndGBM incorporating the Stupp protocol as an arm. For the selected studies, we extracted individual patient survival pseudodata of the Stupp protocol arm by digitizing the Kaplan-Meier plots. A comprehensive pipeline was established to select suitable control studies as external benchmarks.
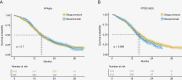
Results: Among the 13 identified studies identified in our systematic review, 4 studies resulted as comparable with the AVAglio trial and 2 with the RTOG 0825. Pooled individual patient pseudodata analysis showed no differences in terms of OS when bevacizumab was added to the Stupp protocol.
Conclusions: The external-controlled-based reassessment of the bevacizumab treatment in ndGBM confirmed its lack of efficacy in extending OS. Our study includes a summary table of individual patient survival pseudodata from all phase 3 RCTs in ndGBM employing the Stupp protocol and provides a pipeline that offers comprehensive guidance for conducting external control-based assessments in ndGBM.
Keywords: Kaplan–Meier digitalization; bevacizumab; external controls; newly diagnosed glioblastoma.
© The Author(s) 2024. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
To the best of our knowledge, none of the authors have conflicts of interest related to the findings or materials presented in this study.
Figures

References
-
- Barnholtz-Sloan JS, Ostrom QT, Cote D.. Epidemiology of brain tumors. Neurol Clin. 2018;36(3):395–419. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. - PubMed
-
- Friedman HS, Prados MD, Wen PY, et al. . Bevacizumab alone and in combination with Irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
