Glycemic control in children with type 1 diabetes treated with the advanced hybrid closed loop system 2-year prospective, observational, two-center study
- PMID: 38390211
- PMCID: PMC10882083
- DOI: 10.3389/fendo.2024.1332418
Glycemic control in children with type 1 diabetes treated with the advanced hybrid closed loop system 2-year prospective, observational, two-center study
Abstract
Background and aims: MiniMed 780G is the first Advanced Hybrid Closed Loop (AHCL) system in Poland, approved in the EU in 2020. To date, observations of glycemic control up to 12 months have been published. This study aimed to analyze glycemic control and anthropometric parameters in children and adolescents with type 1 diabetes (T1D) after two years of using the AHCL system.
Materials and methods: We prospectively collected anthropometric data, pump, and continuous glucose records of fifty T1D children (9.9 ± 2.4 years, 24 (48%) boys, T1D for 3.9 ± 2.56 years) using an AHCL system. We compared the two-week AHCL records obtained after AHCL enrollment with data 6, 12, and 24 months after starting AHCL.
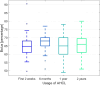
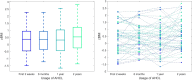
Results: Time in range (70-180 mg/dl) and BMI z-score did not change during the 2 years of observation (p>0.05). The percentage of autocorrection in total daily insulin increased significantly (p<0.005).
Conclusion: Glycemic control in the investigated group of children with T1D treated with the AHCL system for 2 years remained stable. Children in this group maintained weight and optimal metabolic control, most likely due to autocorrection boluses.
Keywords: BMI; advanced hybrid closed-loop system; body mass index; children; time in range; type 1 diabetes.
Copyright © 2024 Seget, Chobot, Tarasiewicz, Bielawska, Rusak, Ochab, Polanska and Jarosz-Chobot.
Conflict of interest statement
PJ-C has received speaker honoraria from Medtronic, Dexcom, Abbott, Ypsomed, and Roche, was a member of the advisory boards for Medtronic and Abbott, and received research support from Medtronic. SS has received speaker honoraria from Medtronic and Ypsomed. The remaining authors declare that the research was conducted without commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Body mass index, basal insulin and glycemic control in children with type 1 diabetes treated with the advanced hybrid closed loop system remain stable - 1-year prospective, observational, two-center study.Front Endocrinol (Lausanne). 2022 Oct 11;13:1036808. doi: 10.3389/fendo.2022.1036808. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36303875 Free PMC article.
-
Glycemic outcomes of Advanced Hybrid Closed Loop system in children and adolescents with Type 1 Diabetes, previously treated with Multiple Daily Injections (MiniMed 780G system in T1D individuals, previously treated with MDI).BMC Endocr Disord. 2022 Mar 29;22(1):80. doi: 10.1186/s12902-022-00996-7. BMC Endocr Disord. 2022. PMID: 35351095 Free PMC article.
-
Real-Life Achievements of MiniMed 780G Advanced Closed-Loop System in Youth with Type 1 Diabetes: AWeSoMe Study Group Multicenter Prospective Trial.Diabetes Technol Ther. 2024 Nov;26(11):869-880. doi: 10.1089/dia.2024.0148. Epub 2024 May 31. Diabetes Technol Ther. 2024. PMID: 38758194
-
Glycemic Control Across the Menstrual Cycle in Women with Type 1 Diabetes Using the MiniMed 780G Advanced Hybrid Closed-Loop System: The 780MENS Prospective Study.Diabetes Technol Ther. 2025 May;27(5):395-401. doi: 10.1089/dia.2024.0522. Epub 2025 Jan 16. Diabetes Technol Ther. 2025. PMID: 39898554
-
Efficacy of advanced hybrid closed loop systems for the management of type 1 diabetes in children.Minerva Pediatr (Torino). 2021 Dec;73(6):474-485. doi: 10.23736/S2724-5276.21.06531-9. Epub 2021 Jul 26. Minerva Pediatr (Torino). 2021. PMID: 34309344 Review.
Cited by
-
Children and adolescent with suboptimal control of type 1 diabetes improve during the first 2 years on automated insulin delivery system.Diabetes Obes Metab. 2025 Jan;27(1):134-142. doi: 10.1111/dom.15992. Epub 2024 Sep 30. Diabetes Obes Metab. 2025. PMID: 39344828 Free PMC article.
-
Long-term use of Minimed™ 780G in children and adolescents with type 1 diabetes under real-world conditions: The benefits of optimal settings.Diabetes Obes Metab. 2025 Apr;27(4):2309-2312. doi: 10.1111/dom.16226. Epub 2025 Jan 31. Diabetes Obes Metab. 2025. PMID: 39887516 Free PMC article. No abstract available.
-
Impact of School Nurse on Managing Pediatric Type 1 Diabetes with Technological Devices Support: A Systematic Review.Diseases. 2024 Aug 1;12(8):173. doi: 10.3390/diseases12080173. Diseases. 2024. PMID: 39195172 Free PMC article. Review.
-
Investigating the Incidence of Dyslipidemia among Brazilian Children and Adolescents Diagnosed with Type 1 Diabetes Mellitus: A Cross-Sectional Study.Diseases. 2024 Feb 24;12(3):45. doi: 10.3390/diseases12030045. Diseases. 2024. PMID: 38534969 Free PMC article.
-
Real world efficacy and safety of the advanced hybrid closed-loop system MiniMed 780G (SmartGuard) in children under 7 years of age.Front Med (Lausanne). 2025 Jan 6;11:1465800. doi: 10.3389/fmed.2024.1465800. eCollection 2024. Front Med (Lausanne). 2025. PMID: 39835094 Free PMC article.
References
-
- Medtronic: MiniMed 780G. System User Guide. Available at: https://www.medtronic-diabetes.co.uk/sites/uk/medtronic-diabetes.co.uk/f... (Accessed January 24, 2022).
-
- Seget S, Rusak E, Polanska J, Jarosz-Chobot P. Prospective open-label, single-arm, single-center follow-up study of the application of the advanced hybrid closed loop system in well-controlled children and adolescents with type 1 diabetes. Diabetes Technol Ther (2022) 24(11):824–31. doi: 10.1089/dia.2022.0148 - DOI - PMC - PubMed
-
- Matejko B, Juza A, Kieć-Wilk B, Cyranka K, Krzyżowska S, Chen X, et al. . Transitioning of people with type 1 diabetes from multiple daily injections and self-monitoring of blood glucose directly to miniMed 780G advanced hybrid closed-loop system: A two-center, randomized, controlled study. Diabetes Care (2022) 45(11):2628–35. doi: 10.2337/dc22-0470 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

