Psychiatric adverse events associated with GLP-1 receptor agonists: a real-world pharmacovigilance study based on the FDA Adverse Event Reporting System database
- PMID: 38390214
- PMCID: PMC10882716
- DOI: 10.3389/fendo.2024.1330936
Psychiatric adverse events associated with GLP-1 receptor agonists: a real-world pharmacovigilance study based on the FDA Adverse Event Reporting System database
Abstract
Background: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are widely used due to their profound efficacy in glycemic control and weight management. Within real-world contexts, the manifestation of certain psychiatric adverse events (AEs) has been observed, which is potentially linked to the administration of GLP-1 RAs. The objective of this study was to undertake a comprehensive investigation and characterization of the psychiatric AEs associated with GLP-1 RAs.
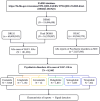
Methods: We retrieved reports of AEs associated with treatment with GLP-1 RAs during the period from the first quarter (Q1) of 2004 to Q1 2023 from the FDA Adverse Event Reporting System (FAERS) database. Descriptive analysis was performed to examine the clinical characteristics and time to onset of the psychiatric AEs caused by GLP-1 RAs. Moreover, disproportionality analyses were performed using the reporting odds ratio (ROR) to identify GLP-1 RA-related psychiatric AEs.
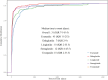
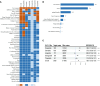
Results: A total of 8,240 reports of psychiatric AEs were analyzed out of 181,238 AE reports with treatment with GLP-1 RAs. Among these cases, a higher percentage was represented by women compared to men (65.89% vs. 30.96%). The median age of these patients was 56 years, with an interquartile range (IQR) of 48-67 years, based on data available in 286 case reports. This study showed that the median time to onset of the overall GLP-1 RA-related AEs was 31 days (IQR = 7-145.4 days), which varied among GLP-1 RA regimens. Specifically, exenatide had a significantly longer onset time at 45 days (IQR = 11-213 days), with statistically significant differences from the onset times of the other five GLP-1 RAs (p< 0.0001). Moreover, eight categories of psychiatric AEs, namely, nervousness (ROR = 1.97, 95% CI = 1.85-2.11), stress (ROR = 1.28, 95% CI = 1.19-1.38), eating disorder (ROR = 1.57, 95% CI = 1.40-1.77), fear of injection (ROR = 1.96, 95% CI = 1.60-2.40), sleep disorder due to general medical condition-insomnia type (ROR = 2.01, 95% CI = 1.60-2.52), binge eating (ROR = 2.70, 95% CI = 1.75-4.16), fear of eating (ROR 3.35, 95% CI = 1.65-6.78), and self-induced vomiting (ROR = 3.77, 95% CI = 1.77-8.03), were defined as GLP-1 RA-related psychiatric AEs through disproportionality analysis.
Conclusion: Our findings demonstrate a significant association between GLP-1 RAs and the development of specific psychiatric AEs. Despite the observational nature of this pharmacovigilance study and the inherent limitations of the FAERS database, our preliminary findings in this work could provide a better basis for understanding the potential psychiatric AEs that may occur with GLP-1 RA treatment, assisting clinicians to focus on these AEs and provide early intervention for optimal risk management.
Keywords: FAERS database; disproportionality analyses; glucagon-like peptide-1 receptor agonists; pharmacovigilance study; psychiatric adverse events.
Copyright © 2024 Chen, Cai, Zou and Fu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Association between different GLP-1 receptor agonists and gastrointestinal adverse reactions: A real-world disproportionality study based on FDA adverse event reporting system database.Front Endocrinol (Lausanne). 2022 Dec 7;13:1043789. doi: 10.3389/fendo.2022.1043789. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36568085 Free PMC article.
-
Neuropsychiatric adverse events associated with Glucagon-like peptide-1 receptor agonists: a pharmacovigilance analysis of the FDA Adverse Event Reporting System database.Eur Psychiatry. 2025 Feb 4;68(1):e20. doi: 10.1192/j.eurpsy.2024.1803. Eur Psychiatry. 2025. PMID: 39901452 Free PMC article.
-
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and suicidality: A replication study using reports to the World Health Organization pharmacovigilance database (VigiBase®).J Affect Disord. 2025 Jan 15;369:922-927. doi: 10.1016/j.jad.2024.10.062. Epub 2024 Oct 19. J Affect Disord. 2025. PMID: 39433133
-
Interstitial lung disease with antibody-drug conjugates: a real-world pharmacovigilance study based on the FAERS database during the period 2014-2023.Ther Adv Respir Dis. 2024 Jan-Dec;18:17534666241299935. doi: 10.1177/17534666241299935. Ther Adv Respir Dis. 2024. PMID: 39660786 Free PMC article.
-
Exploring Connections Between Weight-Loss Medications and Thyroid Cancer: A Look at the FDA Adverse Event Reporting System Database.Endocrinol Diabetes Metab. 2025 Mar;8(2):e70038. doi: 10.1002/edm2.70038. Endocrinol Diabetes Metab. 2025. PMID: 40055991 Free PMC article. Review.
Cited by
-
In Silico Pharmacogenomic Assessment of Glucagon-like Peptide-1 (GLP1) Agonists and the Genetic Addiction Risk Score (GARS) Related Pathways: Implications for Suicidal Ideation and Substance Use Disorder.Curr Neuropharmacol. 2025;23(8):974-995. doi: 10.2174/011570159X349579241231080602. Curr Neuropharmacol. 2025. PMID: 39865816 Free PMC article.
-
A System-Based Review on Effects of Glucagon-Like Peptide-1 Receptor Agonists: Benefits vs Risks.Cureus. 2025 Feb 5;17(2):e78575. doi: 10.7759/cureus.78575. eCollection 2025 Feb. Cureus. 2025. PMID: 40062098 Free PMC article. Review.
-
Effect and mechanism of GLP-1 on cognitive function in diabetes mellitus.Front Neurosci. 2025 Mar 18;19:1537898. doi: 10.3389/fnins.2025.1537898. eCollection 2025. Front Neurosci. 2025. PMID: 40171533 Free PMC article. Review.
-
Glucagon-like Peptide-1 Receptor Agonists in the Context of Eating Disorders: A Promising Therapeutic Option or a Double-Edged Sword?J Clin Med. 2025 Apr 30;14(9):3122. doi: 10.3390/jcm14093122. J Clin Med. 2025. PMID: 40364152 Free PMC article. Review.
-
Dual defense: Opportunities and challenges of GLP-1 receptor agonists in reducing dementia risk in type 2 diabetes!Alzheimers Dement. 2025 May;21(5):e70336. doi: 10.1002/alz.70336. Alzheimers Dement. 2025. PMID: 40442874 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

