Real-world use of first-line pembrolizumab + platinum + taxane combination regimens in recurrent / metastatic head and neck squamous cell carcinoma
- PMID: 38390265
- PMCID: PMC10881782
- DOI: 10.3389/fonc.2024.1348045
Real-world use of first-line pembrolizumab + platinum + taxane combination regimens in recurrent / metastatic head and neck squamous cell carcinoma
Abstract
Introduction: The programmed death-1 (PD-1) immune checkpoint inhibitor pembrolizumab is currently approved in the US for the first-line (1L) treatment of recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC), either alone or in combination with platinum and 5-fluorouracil (5-FU). However, the toxicity of 5-FU has motivated the study of alternate combinations that replace 5-FU with a taxane. The objective of the current study was to describe the baseline characteristics, treatment patterns and sequences, and real-world outcomes of individuals receiving pembrolizumab + platinum + taxane as 1L treatment for R/M HNSCC in the US.
Methods: This was a retrospective study of US adults ≥18 years of age receiving pembrolizumab + platinum + taxane as 1L treatment for R/M HNSCC, using electronic health record data from a nationwide de-identified database. Real-world overall survival (rwOS), time on treatment (rwToT), and time to next treatment (rwTTNT) outcomes were assessed using Kaplan-Meier analysis.
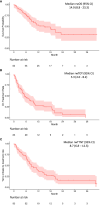
Results: The study population comprised 83 individuals (80.7% male) with a median age of 64 years. The most common tumor site was the oropharynx (48.2%); 70.0% of these tumors were HPV-positive. A total of 71.1% of the study population had an Eastern Cooperative Oncology Group performance status of 0-1 at index date, 71.8% had a combined positive score for programmed death ligand-1 (PD-L1) expression of ≥1, and 30.8% had a score of ≥20. The median (95% CI) rwOS was 14.9 (8.8-23.3) months, rwToT was 5.3 (4.0-8.2) months, and rwTTNT was 8.7 (6.8-12.3) months. Among the 24 individuals who received a subsequent therapy, the most common second-line therapies were cetuximab-based (n = 9) or pembrolizumab-containing (n = 8) regimens.
Conclusions: The rwOS and other real-world outcomes observed for this study population further support pembrolizumab + platinum + taxane combination therapy as a potential 1L treatment option for R/M HNSCC.
Keywords: Kaplan-Meier estimate; PD-1; antineoplastic agents; head and neck squamous cell carcinoma; patient outcomes; real-world observational study; taxanes; treatment patterns.
Copyright © 2024 Black, Zheng, Hair, Ai, Wang, Goto, Lerman, Bidadi and Hanna.
Conflict of interest statement
Authors CB, DZ, GMH, LA, LW, NL, and BB were employed by the company Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may be shareholders in Merck & Co., Inc., Rahway, NJ, USA. Author DG was employed by the company Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and a shareholder in Merck & Co., Inc., Rahway, NJ, USA at the time of the study. Author GJH has received consulting fees from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Figures
Similar articles
-
Real-world treatment patterns and outcomes among individuals receiving first-line pembrolizumab therapy for recurrent/metastatic head and neck squamous cell carcinoma.Front Oncol. 2023 May 22;13:1160144. doi: 10.3389/fonc.2023.1160144. eCollection 2023. Front Oncol. 2023. PMID: 37284189 Free PMC article.
-
Global treatment patterns and outcomes among patients with recurrent and/or metastatic head and neck squamous cell carcinoma: Results of the GLANCE H&N study.Oral Oncol. 2020 Mar;102:104526. doi: 10.1016/j.oraloncology.2019.104526. Epub 2020 Jan 21. Oral Oncol. 2020. PMID: 31978755
-
PD-L1 testing patterns in recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) in the U.S.Oral Oncol. 2025 Feb;161:107146. doi: 10.1016/j.oraloncology.2024.107146. Epub 2025 Jan 3. Oral Oncol. 2025. PMID: 39754998
-
Pembrolizumab in the first-line treatment of advanced head and neck cancer.Expert Rev Anticancer Ther. 2021 Dec;21(12):1321-1331. doi: 10.1080/14737140.2021.1996228. Epub 2021 Nov 2. Expert Rev Anticancer Ther. 2021. PMID: 34689660 Review.
-
The Evolving Role of Taxanes in Combination With Cetuximab for the Treatment of Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck: Evidence, Advantages, and Future Directions.Front Oncol. 2019 Aug 21;9:668. doi: 10.3389/fonc.2019.00668. eCollection 2019. Front Oncol. 2019. PMID: 31497530 Free PMC article. Review.
Cited by
-
Immunotherapy for primary squamous cell carcinoma of the liver: A case report.Oncol Lett. 2025 Apr 7;29(6):279. doi: 10.3892/ol.2025.15025. eCollection 2025 Jun. Oncol Lett. 2025. PMID: 40242270 Free PMC article.
-
The efficacy and safety of a taxane-based chemotherapy regimen combined with a PD-1 inhibitor in HNSCC: a multicenter real-world study.World J Surg Oncol. 2025 Jan 4;23(1):6. doi: 10.1186/s12957-024-03644-7. World J Surg Oncol. 2025. PMID: 39754263 Free PMC article.
-
Pembrolizumab with Carboplatin and Paclitaxel Versus Alternative Systemic Treatments Recommended for the First-Line Treatment of Recurrent/Metastatic Head and Neck Cancer: An Indirect Treatment Comparison.Adv Ther. 2025 Jun;42(6):2690-2707. doi: 10.1007/s12325-025-03144-4. Epub 2025 Apr 24. Adv Ther. 2025. PMID: 40272712
References
-
- Head and neck cancer: Statistics. ASCO; Last Updated: February 2023. https://www.cancer.net/cancer-types/head-and-neck-cancer/statistics (Accessed January 18, 2024).
LinkOut - more resources
Full Text Sources
Research Materials