The TRPM2 ion channel regulates metabolic and thermogenic adaptations in adipose tissue of cold-exposed mice
- PMID: 38390373
- PMCID: PMC10882718
- DOI: 10.3389/fendo.2023.1251351
The TRPM2 ion channel regulates metabolic and thermogenic adaptations in adipose tissue of cold-exposed mice
Abstract
Introduction: During thermogenesis, adipose tissue (AT) becomes more active and enhances oxidative metabolism. The promotion of this process in white AT (WAT) is called "browning" and, together with the brown AT (BAT) activation, is considered as a promising approach to counteract obesity and metabolic diseases. Transient receptor potential cation channel, subfamily M, member 2 (TRPM2), is an ion channel that allows extracellular Ca2+ influx into the cytosol, and is gated by adenosine diphosphate ribose (ADPR), produced from NAD+ degradation. The aim of this study was to investigate the relevance of TRPM2 in the regulation of energy metabolism in BAT, WAT, and liver during thermogenesis.
Methods: Wild type (WT) and Trpm2-/- mice were exposed to 6°C and BAT, WAT and liver were collected to evaluate mRNA, protein levels and ADPR content. Furthermore, O2 consumption, CO2 production and energy expenditure were measured in these mice upon thermogenic stimulation. Finally, the effect of the pharmacological inhibition of TRPM2 was assessed in primary adipocytes, evaluating the response upon stimulation with the β-adrenergic receptor agonist CL316,243.
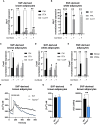
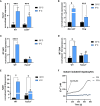
Results: Trpm2-/- mice displayed lower expression of browning markers in AT and lower energy expenditure in response to thermogenic stimulus, compared to WT animals. Trpm2 gene overexpression was observed in WAT, BAT and liver upon cold exposure. In addition, ADPR levels and mono/poly-ADPR hydrolases expression were higher in mice exposed to cold, compared to control mice, likely mediating ADPR generation.
Discussion: Our data indicate TRPM2 as a fundamental player in BAT activation and WAT browning. TRPM2 agonists may represent new pharmacological strategies to fight obesity.
Keywords: ADPr; TRPM2; brown adipose tissue; browning; cold exposure; thermogenesis; white adipose tissue.
Copyright © 2023 Benzi, Heine, Spinelli, Salis, Worthmann, Diercks, Astigiano, Pérez Mato, Memushaj, Sturla, Vellone, Damonte, Jaeckstein, Koch-Nolte, Mittrücker, Guse, De Flora, Heeren and Bruzzone.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

