Stereochemical insights into β-amino- N-acylhydrazones and their impact on DPP-4 inhibition
- PMID: 38390500
- PMCID: PMC10882265
- DOI: 10.1039/d4ra00450g
Stereochemical insights into β-amino- N-acylhydrazones and their impact on DPP-4 inhibition
Abstract
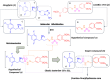
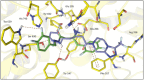
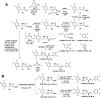
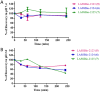
Dipeptidyl peptidase IV (DPP-4) is a key enzyme that regulates several important biological processes and it is better known to be targeted by gliptins as a modern validated approach for the management of type 2 diabetes mellitus (T2DM). However, new generations of DPP-4 inhibitors capable of controlling inflammatory processes associated with chronic complications of T2DM are still needed. In this scenario, we report here the design by molecular modelling of new β-amino-N-acylhydrazones, their racemic synthesis, chiral resolution, determination of physicochemical properties and their DPP4 inhibitory potency. Theoretical and experimental approaches allowed us to propose a preliminary SAR, as well as to identify LASSBio-2124 (6) as a new lead for DPP-4 inhibition, with good physicochemical properties, favourable eudismic ratio, scalable synthesis and anti-diabetes effect in a proof-of-concept model. These findings represent an interesting starting point for the development of a new generation of DPP-4 inhibitors, useful in the treatment of T2DM and comorbidities.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

