Autophagy: a necessary evil in cancer and inflammation
- PMID: 38390576
- PMCID: PMC10879063
- DOI: 10.1007/s13205-023-03864-w
Autophagy: a necessary evil in cancer and inflammation
Abstract
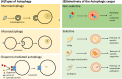
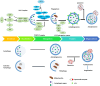
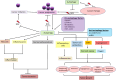
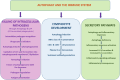
Autophagy, a highly regulated cellular process, assumes a dual role in the context of cancer. On the one hand, it functions as a crucial homeostatic pathway, responsible for degrading malfunctioning molecules and organelles, thereby maintaining cellular health. On the other hand, its involvement in cancer development and regression is multifaceted, contingent upon a myriad of factors. This review meticulously examines the intricacies of autophagy, from its molecular machinery orchestrated by Autophagy-Related Genes (ATG) initially discovered in yeast to the various modes of autophagy operative within cells. Beyond its foundational role in cellular maintenance, autophagy reveals context-specific functions in processes like angiogenesis and inflammation. Our analysis delves into how autophagy-related factors directly impact inflammation, underscoring their profound implications for cancer dynamics. Additionally, we extend our inquiry to explore autophagy's associations with cardiovascular conditions, neurodegenerative disorders, and autoimmune diseases, illuminating the broader medical relevance of this process. Furthermore, this review elucidates how autophagy contributes to sustaining hallmark cancer features, including stem cell maintenance, proliferation, angiogenesis, metastasis, and metabolic reprogramming. Autophagy emerges as a pivotal process that necessitates careful consideration in cancer treatment strategies. To this end, we investigate innovative approaches, ranging from enzyme-based therapies to MTOR inhibitors, lysosomal blockers, and nanoparticle-enabled interventions, all aimed at optimizing cancer treatment outcomes by targeting autophagy pathways. In summary, this comprehensive review provides a nuanced perspective on the intricate and context-dependent role of autophagy in cancer biology. Our exploration not only deepens our understanding of this fundamental process but also highlights its potential as a therapeutic target. By unraveling the complex interplay between autophagy and cancer, we pave the way for more precise and effective cancer treatments, promising better outcomes for patients.
Keywords: Angiogenesis; Autophagy; Cancer; Endoplasmic reticulum; Inflammation; Nanoparticle.
© King Abdulaziz City for Science and Technology 2024. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

