Interim 2023/24 influenza A vaccine effectiveness: VEBIS European primary care and hospital multicentre studies, September 2023 to January 2024
- PMID: 38390651
- PMCID: PMC10899813
- DOI: 10.2807/1560-7917.ES.2024.29.8.2400089
Interim 2023/24 influenza A vaccine effectiveness: VEBIS European primary care and hospital multicentre studies, September 2023 to January 2024
Abstract
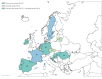
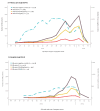
Influenza A viruses circulated in Europe from September 2023 to January 2024, with influenza A(H1N1)pdm09 predominance. We provide interim 2023/24 influenza vaccine effectiveness (IVE) estimates from two European studies, covering 10 countries across primary care (EU-PC) and hospital (EU-H) settings. Interim IVE was higher against A(H1N1)pdm09 than A(H3N2): EU-PC influenza A(H1N1)pdm09 IVE was 53% (95% CI: 41 to 63) and 30% (95% CI: -3 to 54) against influenza A(H3N2). For EU-H, these were 44% (95% CI: 30 to 55) and 14% (95% CI: -32 to 43), respectively.
Keywords: Europe; influenza; multicentre study; test-negative design; vaccine effectiveness.
Conflict of interest statement
Figures
References
-
- European Centre for Disease Prevention and Control (ECDC). European Respiratory Virus Surveillance Summary (ERVISS), 2024, Week 05. Stockholm: ECDC. [Accessed: 16 Feb 2024]. Available from: https://erviss.org
-
- World Health Organization (WHO). Recommended composition of influenza virus vaccines for use in the 2023-2024 northern hemisphere influenza season. Geneva: WHO; 2023. Available from: https://www.who.int/publications/m/item/recommended-composition-of-influ...
-
- European Centre for Disease Prevention and Control (ECDC). Vaccine Scheduler. Stockholm: ECDC. [Accessed: 31 Jan 2024]. Available from: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDise...
-
- European Centre for Disease Prevention and Control (ECDC). Core protocol for ECDC studies of vaccine effectiveness against symptomatic laboratory-confirmed influenza or SARS-CoV-2 infection at primary care level: version 1.0, September 2023. Stockholm: ECDC; 2023. Available from: https://data.europa.eu/doi/10.2900/25966 - DOI
-
- European Centre for Disease Prevention and Control (ECDC). Core protocol for ECDC VEBIS studies of COVID-19 vaccine effectiveness against hospitalisation with Severe Acute Respiratory Infection laboratory-confirmed with SARS-CoV-2 or seasonal influenza - Version 2.0. Stockholm: ECDC; 2023. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-vaccin...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
