Early Developmental Exposure to Triclosan Impacts Fecal Microbial Populations, IgA and Functional Activities of the Rat Microbiome
- PMID: 38390992
- PMCID: PMC10885032
- DOI: 10.3390/jox14010012
Early Developmental Exposure to Triclosan Impacts Fecal Microbial Populations, IgA and Functional Activities of the Rat Microbiome
Abstract
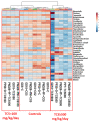
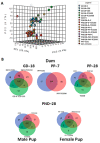
Triclosan (TCS), a broad-spectrum antibacterial chemical, is detected in human urine, breast milk, amniotic fluid, and feces; however, little is known about its impact on the intestinal microbiome and host mucosal immunity during pregnancy and early development. Pregnant female rats were orally gavaged with TCS from gestation day (GD) 6 to postpartum (PP) day 28. Offspring were administered TCS from postnatal day (PND) 12 to 28. Studies were conducted to assess changes in the intestinal microbial population (16S-rRNA sequencing) and functional analysis of microbial genes in animals exposed to TCS during pregnancy (GD18), and at PP7, PP28 and PND28. Microbial abundance was compared with the amounts of TCS excreted in feces and IgA levels in feces. The results reveal that TCS decreases the abundance of Bacteroidetes and Firmicutes with a significant increase in Proteobacteria. At PND28, total Operational Taxonomic Units (OTUs) were higher in females and showed correlation with the levels of TCS and unbound IgA in feces. The significant increase in Proteobacteria in all TCS-treated rats along with the increased abundance in OTUs that belong to pathogenic bacterial communities could serve as a signature of TCS-induced dysbiosis. In conclusion, TCS can perturb the microbiome, the functional activities of the microbiome, and activate mucosal immunity during pregnancy and early development.
Keywords: antimicrobial; endocrine disrupting chemical; fecal IgA; gastrointestinal tract; microbiome; oral exposure; perinatal exposure; postnatal exposure; risk assessment; toxicity; triclosan; xenobiotic.
Conflict of interest statement
The findings and conclusions presented in this manuscript are those of the authors and do not necessarily represent the views of the U.S. Food and Drug Administration or the National Institutes of Health. The authors declare no conflicts of interest.
Figures







References
-
- Food and Drug Adminnistration Safety and effectiveness of consumer antiseptics: Topical antimicrobial drug products for over-the-counter human use. J. Fed. Regist. 2016;81:61106–61130. - PubMed
-
- Food and Drug Adminnistration Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use; Proposed Amendment of the Tentative Final Monograph; Reopening of Administrative Record. Fed. Regist. 2016;21:42911–42937. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

