HER2+ advanced gastric cancer: Current state and opportunities (Review)
- PMID: 38391024
- PMCID: PMC10901538
- DOI: 10.3892/ijo.2024.5624
HER2+ advanced gastric cancer: Current state and opportunities (Review)
Abstract
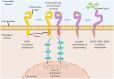
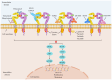
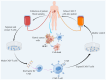
Human epidermal growth factor receptor 2 (HER2)+ gastric cancer (GC) is a distinct subtype of GC, accounting for 10‑20% of all cases of GC. Although the development of the anti‑HER2 monoclonal antibody trastuzumab has markedly improved response rates and prognosis of patients with HER2+ advanced GC (AGC), drug resistance remains a considerable challenge. Therefore, dynamic monitoring of HER2 expression levels can facilitate the identification of patients who may benefit from targeted therapy. Besides trastuzumab, DS‑8201 and RC48 have been applied in the treatment of HER2+ AGC, and several novel anti‑HER2 therapies are undergoing preclinical/clinical trials. At present, combination immunotherapy with anti‑HER2 agents is used as the first‑line treatment of this disease subtype. New promising approaches such as chimeric antigen receptor T‑cell immunotherapy and cancer vaccines are also being investigated for their potential to improve clinical outcomes. The current review provides new insights that will guide the future application of anti‑HER2 therapy by summarizing research progress on targeted therapy drugs for HER2+ AGC and combination treatments.
Keywords: advanced gastric cancer; human epidermal growth factor receptor 2; immunotherapy; targeted therapy; trastuzumab.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Human Epidermal Growth Factor Receptor 2 Positive Advanced Gastric or Esophagogastric Adenocarcinoma: Reflecting on the Past to Gain a New Insights.Curr Oncol Rep. 2025 Jan;27(1):15-29. doi: 10.1007/s11912-024-01626-2. Epub 2025 Jan 3. Curr Oncol Rep. 2025. PMID: 39753814 Review.
-
HER2-Positive Gastric Cancer: The Role of Immunotherapy and Novel Therapeutic Strategies.Int J Mol Sci. 2023 Jul 13;24(14):11403. doi: 10.3390/ijms241411403. Int J Mol Sci. 2023. PMID: 37511163 Free PMC article. Review.
-
Evolving Landscape of HER2-Targeted Therapies for Gastric Cancer Patients.Curr Treat Options Oncol. 2025 Apr;26(4):260-277. doi: 10.1007/s11864-025-01300-0. Epub 2025 Mar 8. Curr Treat Options Oncol. 2025. PMID: 40056280 Review.
-
A critical review of HER2-positive gastric cancer evaluation and treatment: from trastuzumab, and beyond.Cancer Lett. 2014 Aug 28;351(1):30-40. doi: 10.1016/j.canlet.2014.05.019. Epub 2014 Jun 3. Cancer Lett. 2014. PMID: 24943493 Review.
-
Porphyrin modified trastuzumab improves efficacy of HER2 targeted photodynamic therapy of gastric cancer.Int J Cancer. 2017 Oct 1;141(7):1478-1489. doi: 10.1002/ijc.30844. Epub 2017 Jul 10. Int J Cancer. 2017. PMID: 28639285
Cited by
-
Prognosis and Treatment of Gastric Cancer: A 2024 Update.Cancers (Basel). 2024 Apr 27;16(9):1708. doi: 10.3390/cancers16091708. Cancers (Basel). 2024. PMID: 38730659 Free PMC article. Review.
-
Dronedarone hydrochloride inhibits gastric cancer proliferation in vitro and in vivo by targeting SRC.Transl Oncol. 2024 Dec;50:102136. doi: 10.1016/j.tranon.2024.102136. Epub 2024 Oct 5. Transl Oncol. 2024. PMID: 39369581 Free PMC article.
-
Deep learning application in prediction of cancer molecular alterations based on pathological images: a bibliographic analysis via CiteSpace.J Cancer Res Clin Oncol. 2024 Oct 18;150(10):467. doi: 10.1007/s00432-024-05992-z. J Cancer Res Clin Oncol. 2024. PMID: 39422817 Free PMC article.
-
Changes in thyroid hormone levels indicate immunotherapy efficacy in gastric cancer.Oncol Lett. 2025 May 26;30(1):364. doi: 10.3892/ol.2025.15110. eCollection 2025 Jul. Oncol Lett. 2025. PMID: 40469917 Free PMC article.
-
Targeting LINC00665/miR-199b-5p/SERPINE1 axis to inhibit trastuzumab resistance and tumorigenesis of gastric cancer via PI3K/AKt pathway.Noncoding RNA Res. 2024 Jul 31;10:153-162. doi: 10.1016/j.ncrna.2024.07.004. eCollection 2025 Feb. Noncoding RNA Res. 2024. PMID: 39399377 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram L, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Marano L, Roviello F. The distinctive nature of HER2-positive gastric cancers. Eur J Surg Oncol. 2015;41:271–273. - PubMed
-
- Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das R Enzinger PC, Enzler T, Fanta R, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:167–192. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
