Quantitative US Delta Radiomics to Predict Radiation Response in Individuals with Head and Neck Squamous Cell Carcinoma
- PMID: 38391311
- PMCID: PMC10988345
- DOI: 10.1148/rycan.230029
Quantitative US Delta Radiomics to Predict Radiation Response in Individuals with Head and Neck Squamous Cell Carcinoma
Abstract
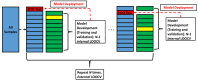
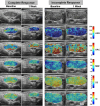
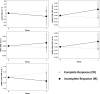
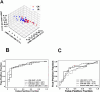
Purpose To investigate the role of quantitative US (QUS) radiomics data obtained after the 1st week of radiation therapy (RT) in predicting treatment response in individuals with head and neck squamous cell carcinoma (HNSCC). Materials and Methods This prospective study included 55 participants (21 with complete response [median age, 65 years {IQR: 47-80 years}, 20 male, one female; and 34 with incomplete response [median age, 59 years {IQR: 39-79 years}, 33 male, one female) with bulky node-positive HNSCC treated with curative-intent RT from January 2015 to October 2019. All participants received 70 Gy of radiation in 33-35 fractions over 6-7 weeks. US radiofrequency data from metastatic lymph nodes were acquired prior to and after 1 week of RT. QUS analysis resulted in five spectral maps from which mean values were extracted. We applied a gray-level co-occurrence matrix technique for textural analysis, leading to 20 QUS texture and 80 texture-derivative parameters. The response 3 months after RT was used as the end point. Model building and evaluation utilized nested leave-one-out cross-validation. Results Five delta (Δ) parameters had statistically significant differences (P < .05). The support vector machines classifier achieved a sensitivity of 71% (15 of 21), a specificity of 76% (26 of 34), a balanced accuracy of 74%, and an area under the receiver operating characteristic curve of 0.77 on the test set. For all the classifiers, the performance improved after the 1st week of treatment. Conclusion A QUS Δ-radiomics model using data obtained after the 1st week of RT from individuals with HNSCC predicted response 3 months after treatment completion with reasonable accuracy. Keywords: Computer-Aided Diagnosis (CAD), Ultrasound, Radiation Therapy/Oncology, Head/Neck, Radiomics, Quantitative US, Radiotherapy, Head and Neck Squamous Cell Carcinoma, Machine Learning Clinicaltrials.gov registration no. NCT03908684 Supplemental material is available for this article. © RSNA, 2024.
Keywords: Computer-Aided Diagnosis (CAD); Head and Neck Squamous Cell Carcinoma; Head/Neck; Machine Learning; Quantitative US; Radiation Therapy/Oncology; Radiomics; Radiotherapy; Ultrasound.
Conflict of interest statement
Figures

Similar articles
-
Radiomics-based prediction of T cell-inflamed gene expression profile and prognosis in head and neck squamous cell carcinoma.Med Phys. 2025 Aug;52(8):e18028. doi: 10.1002/mp.18028. Med Phys. 2025. PMID: 40781753
-
Ultrasound delta-radiomics during radiotherapy to predict recurrence in patients with head and neck squamous cell carcinoma.Clin Transl Radiat Oncol. 2021 Mar 12;28:62-70. doi: 10.1016/j.ctro.2021.03.002. eCollection 2021 May. Clin Transl Radiat Oncol. 2021. PMID: 33778174 Free PMC article.
-
Quantitative ultrasound radiomics in predicting recurrence for patients with node-positive head-neck squamous cell carcinoma treated with radical radiotherapy.Cancer Med. 2021 Apr;10(8):2579-2589. doi: 10.1002/cam4.3634. Epub 2020 Dec 13. Cancer Med. 2021. PMID: 33314716 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Minimally invasive surgery versus radiotherapy/chemoradiotherapy for small-volume primary oropharyngeal carcinoma.Cochrane Database Syst Rev. 2016 Dec 11;12(12):CD010963. doi: 10.1002/14651858.CD010963.pub2. Cochrane Database Syst Rev. 2016. PMID: 27943254 Free PMC article.
Cited by
-
Radiomic Model Associated with Tumor Microenvironment Predicts Immunotherapy Response and Prognosis in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma.Research (Wash D C). 2025 Jun 24;8:0749. doi: 10.34133/research.0749. eCollection 2025. Research (Wash D C). 2025. PMID: 40556946 Free PMC article.
References
-
- Lambin P , Leijenaar RTH , Deist TM , et al. . Radiomics: the bridge between medical imaging and personalized medicine . Nat Rev Clin Oncol 2017. ; 14 ( 12 ): 749 – 762 . - PubMed
-
- Mamou J , Oelze ML , eds. Quantitative ultrasound in soft tissues . advances in experimental medicine and biology series . 2nd ed. Springer; , 2023. .
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

