Emerging Issues and Initial Insights into Bacterial Biofilms: From Orthopedic Infection to Metabolomics
- PMID: 38391570
- PMCID: PMC10885942
- DOI: 10.3390/antibiotics13020184
Emerging Issues and Initial Insights into Bacterial Biofilms: From Orthopedic Infection to Metabolomics
Abstract
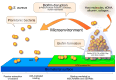
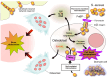
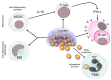
Bacterial biofilms, enigmatic communities of microorganisms enclosed in an extracellular matrix, still represent an open challenge in many clinical contexts, including orthopedics, where biofilm-associated bone and joint infections remain the main cause of implant failure. This study explores the scenario of biofilm infections, with a focus on those related to orthopedic implants, highlighting recently emerged substantial aspects of the pathogenesis and their potential repercussions on the clinic, as well as the progress and gaps that still exist in the diagnostics and management of these infections. The classic mechanisms through which biofilms form and the more recently proposed new ones are depicted. The ways in which bacteria hide, become impenetrable to antibiotics, and evade the immune defenses, creating reservoirs of bacteria difficult to detect and reach, are delineated, such as bacterial dormancy within biofilms, entry into host cells, and penetration into bone canaliculi. New findings on biofilm formation with host components are presented. The article also delves into the emerging and critical concept of immunometabolism, a key function of immune cells that biofilm interferes with. The growing potential of biofilm metabolomics in the diagnosis and therapy of biofilm infections is highlighted, referring to the latest research.
Keywords: bacterial dormancy; biofilm; canaliculo-lacunar network reservoir; immunometabolism; internalization; metabolomics; orthopedic implant infections.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Smart Nanomaterials for Treatment of Biofilm in Orthopedic Implants.Front Bioeng Biotechnol. 2021 Sep 13;9:694635. doi: 10.3389/fbioe.2021.694635. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34589470 Free PMC article. Review.
-
The role of bacterial biofilms in chronic infections.APMIS Suppl. 2013 May;(136):1-51. doi: 10.1111/apm.12099. APMIS Suppl. 2013. PMID: 23635385
-
Bioengineering Approaches to Fight against Orthopedic Biomaterials Related-Infections.Int J Mol Sci. 2022 Oct 1;23(19):11658. doi: 10.3390/ijms231911658. Int J Mol Sci. 2022. PMID: 36232956 Free PMC article. Review.
-
Interactions of Neutrophils with the Polymeric Molecular Components of the Biofilm Matrix in the Context of Implant-Associated Bone and Joint Infections.Int J Mol Sci. 2023 Dec 1;24(23):17042. doi: 10.3390/ijms242317042. Int J Mol Sci. 2023. PMID: 38069365 Free PMC article. Review.
-
The giant staphylococcal protein Embp facilitates colonization of surfaces through Velcro-like attachment to fibrillated fibronectin.Elife. 2022 Jul 7;11:e76164. doi: 10.7554/eLife.76164. Elife. 2022. PMID: 35796649 Free PMC article.
Cited by
-
Staphylococcus aureus Proteins Implicated in the Reduced Virulence of sarA and sarA/agr Mutants in Osteomyelitis.Microorganisms. 2025 Jan 16;13(1):181. doi: 10.3390/microorganisms13010181. Microorganisms. 2025. PMID: 39858949 Free PMC article.
-
Biofilm Formation in Chronic Infections: A Comprehensive Review of Pathogenesis, Clinical Implications, and Novel Therapeutic Approaches.Cureus. 2024 Oct 1;16(10):e70629. doi: 10.7759/cureus.70629. eCollection 2024 Oct. Cureus. 2024. PMID: 39483571 Free PMC article. Review.
-
Assessing Cytotoxicity, Proteolytic Stability, and Selectivity of Antimicrobial Peptides: Implications for Orthopedic Applications.Int J Mol Sci. 2024 Dec 10;25(24):13241. doi: 10.3390/ijms252413241. Int J Mol Sci. 2024. PMID: 39769006 Free PMC article.
-
Research and Application of Chitosan Nanoparticles in Orthopedic Infections.Int J Nanomedicine. 2024 Jul 2;19:6589-6602. doi: 10.2147/IJN.S468848. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38979535 Free PMC article. Review.
-
A unique combination of natural fatty acids from Hermetia illucens fly larvae fat effectively combats virulence factors and biofilms of MDR hypervirulent mucoviscus Klebsiella pneumoniae strains by increasing Lewis acid-base/van der Waals interactions in bacterial wall membranes.Front Cell Infect Microbiol. 2024 Jul 25;14:1408179. doi: 10.3389/fcimb.2024.1408179. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39119288 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

