Silk Fibroin Materials: Biomedical Applications and Perspectives
- PMID: 38391652
- PMCID: PMC10886036
- DOI: 10.3390/bioengineering11020167
Silk Fibroin Materials: Biomedical Applications and Perspectives
Abstract
The golden rule in tissue engineering is the creation of a synthetic device that simulates the native tissue, thus leading to the proper restoration of its anatomical and functional integrity, avoiding the limitations related to approaches based on autografts and allografts. The emergence of synthetic biocompatible materials has led to the production of innovative scaffolds that, if combined with cells and/or bioactive molecules, can improve tissue regeneration. In the last decade, silk fibroin (SF) has gained attention as a promising biomaterial in regenerative medicine due to its enhanced bio/cytocompatibility, chemical stability, and mechanical properties. Moreover, the possibility to produce advanced medical tools such as films, fibers, hydrogels, 3D porous scaffolds, non-woven scaffolds, particles or composite materials from a raw aqueous solution emphasizes the versatility of SF. Such devices are capable of meeting the most diverse tissue needs; hence, they represent an innovative clinical solution for the treatment of bone/cartilage, the cardiovascular system, neural, skin, and pancreatic tissue regeneration, as well as for many other biomedical applications. The present narrative review encompasses topics such as (i) the most interesting features of SF-based biomaterials, bare SF's biological nature and structural features, and comprehending the related chemo-physical properties and techniques used to produce the desired formulations of SF; (ii) the different applications of SF-based biomaterials and their related composite structures, discussing their biocompatibility and effectiveness in the medical field. Particularly, applications in regenerative medicine are also analyzed herein to highlight the different therapeutic strategies applied to various body sectors.
Keywords: 3D scaffolds; biomaterials; biomedicine; bone regeneration; fibroin; films; hydrogels; regenerative medicine; silk; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

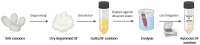


References
-
- Pihlman H., Keränen P., Paakinaho K., Linden J., Hannula M., Manninen I.K., Hyttinen J., Manninen M., Laitinen-Vapaavuori O. Novel Osteoconductive β-Tricalcium Phosphate/Poly(L-Lactide-Co-e-Caprolactone) Scaffold for Bone Regeneration: A Study in a Rabbit Calvarial Defect. J. Mater. Sci. Mater. Med. 2018;29:156. doi: 10.1007/s10856-018-6159-9. - DOI - PubMed
-
- Lai Y., Cao H., Wang X., Chen S., Zhang M., Wang N., Yao Z., Dai Y., Xie X., Zhang P., et al. Porous Composite Scaffold Incorporating Osteogenic Phytomolecule Icariin for Promoting Skeletal Regeneration in Challenging Osteonecrotic Bone in Rabbits. Biomaterials. 2018;153:1–13. doi: 10.1016/j.biomaterials.2017.10.025. - DOI - PubMed
-
- Park H., Kim J.S., Oh E.J., Kim T.J., Kim H.M., Shim J.H., Yoon W.S., Huh J.B., Moon S.H., Kang S.S., et al. Effects of Three-Dimensionally Printed Polycaprolactone/β-Tricalcium Phosphate Scaffold on Osteogenic Differentiation of Adipose Tissue- and Bone Marrow-Derived Stem Cells. Arch. Craniofac. Surg. 2018;19:181–189. doi: 10.7181/acfs.2018.01879. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

