Intra-Articular Injection of Adipose-Derived Stromal Vascular Fraction in Osteoarthritic Temporomandibular Joints: Study Design of a Randomized Controlled Clinical Trial
- PMID: 38391657
- PMCID: PMC10886020
- DOI: 10.3390/bioengineering11020171
Intra-Articular Injection of Adipose-Derived Stromal Vascular Fraction in Osteoarthritic Temporomandibular Joints: Study Design of a Randomized Controlled Clinical Trial
Abstract
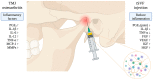
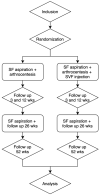
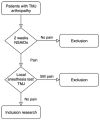
Introduction: Temporomandibular joint (TMJ) osteoarthritis is a degenerative disease of the TMJ. It is characterized by progressive degradation of the extracellular matrix components of articular cartilage, with secondary inflammatory components leading to pain in the temporomandibular region and reduced mouth opening. Current treatments do not halt disease progression, hence the need for new therapies to reduce inflammation and, consequently, improve symptoms. The aim of our randomized controlled clinical trial protocol is to investigate the efficacy of adjuvant intra-articular injections of autologous tissue-like stromal vascular fraction (tSVF), compared to arthrocentesis alone, in reducing pain and improving mouth opening in TMJ osteoarthritis patients.
Materials and methods: The primary endpoint analysis will consist of the visual analogue scale (VAS) for pain. The secondary endpoint analyses will include maximal interincisal mouth opening measurements; assessment of oral health and mandibular function based on the oral health impact profile (OHIP) questionnaire and mandibular functional impairment questionnaire (MFIQ); complications during the follow up; synovial cytokine analysis at baseline and after 26 weeks; and nucleated cells and tSVF (immuno)histochemistry analyses of the intervention group.
Discussion: Our randomized clinical trial protocol will be applied to evaluate the efficacy of a new promising tSVF injection therapy for TMJ osteoarthritis. The safety of intra-articular injections of tSVF has been proven for knee osteoarthritis. However, since a tSVF injection is considered a heterologous application of cell therapy, the regulatory requirements are strict, which makes medical ethical approval challenging.
Keywords: adipose derived stromal cells; adipose tissue; intra-articular injection; temporomandibular joint osteoarthritis; tissue stromal vascular fraction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Autologous Adipose-Derived Tissue Stromal Vascular Fraction (AD-tSVF) for Knee Osteoarthritis.Int J Mol Sci. 2022 Nov 4;23(21):13517. doi: 10.3390/ijms232113517. Int J Mol Sci. 2022. PMID: 36362308 Free PMC article. Review.
-
Does intra-articular injection of tenoxicam after arthrocentesis heal outcomes of temporomandibular joint osteoarthritis? A randomized clinical trial.BMC Oral Health. 2023 Mar 8;23(1):131. doi: 10.1186/s12903-023-02852-z. BMC Oral Health. 2023. PMID: 36890529 Free PMC article. Clinical Trial.
-
Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial.Int Orthop. 2019 May;43(5):1123-1134. doi: 10.1007/s00264-018-4099-0. Epub 2018 Aug 14. Int Orthop. 2019. PMID: 30109404 Clinical Trial.
-
Does Injection of Corticosteroid After Arthrocentesis Improve Outcomes of Temporomandibular Joint Osteoarthritis? A Randomized Clinical Trial.J Oral Maxillofac Surg. 2016 Nov;74(11):2151-2158. doi: 10.1016/j.joms.2016.05.027. Epub 2016 Jun 12. J Oral Maxillofac Surg. 2016. PMID: 27376184 Clinical Trial.
-
Intra-articular Injection of Autologous Adipose-Derived Stem Cells or Stromal Vascular Fractions: Are They Effective for Patients With Knee Osteoarthritis? A Systematic Review With Meta-analysis of Randomized Controlled Trials.Am J Sports Med. 2023 Mar;51(3):837-848. doi: 10.1177/03635465211053893. Epub 2022 Jan 12. Am J Sports Med. 2023. PMID: 35019764
Cited by
-
Elliptical Lipoaspirate Activation Versus Coleman Technique: A Randomized Double-Blinded Clinical Trial on Adipose Tissue Grafting for Scar Treatment.Aesthetic Plast Surg. 2025 May;49(10):2783-2798. doi: 10.1007/s00266-025-04804-0. Epub 2025 Apr 7. Aesthetic Plast Surg. 2025. PMID: 40195135 Clinical Trial.
References
-
- Jerjes W., Upile T., Abbas S., Kafas P., Vourvachis M., Rob J., Mc Carthy E., Angouridakis N., Hopper C. Muscle Disorders and Dentition-Related Aspects in Temporomandibular Disorders: Controversies in the Most Commonly Used Treatment Modalities. Int. Arch. Med. 2008;1:23. doi: 10.1186/1755-7682-1-23. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources