Overview of the Current Knowledge and Conventional MRI Characteristics of Peri- and Para-Vascular Spaces
- PMID: 38391713
- PMCID: PMC10886993
- DOI: 10.3390/brainsci14020138
Overview of the Current Knowledge and Conventional MRI Characteristics of Peri- and Para-Vascular Spaces
Abstract
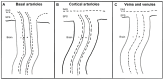
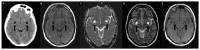
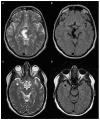
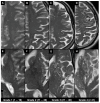
Brain spaces around (perivascular spaces) and alongside (paravascular or Virchow-Robin spaces) vessels have gained significant attention in recent years due to the advancements of in vivo imaging tools and to their crucial role in maintaining brain health, contributing to the anatomic foundation of the glymphatic system. In fact, it is widely accepted that peri- and para-vascular spaces function as waste clearance pathways for the brain for materials such as ß-amyloid by allowing exchange between cerebrospinal fluid and interstitial fluid. Visible brain spaces on magnetic resonance imaging are often a normal finding, but they have also been associated with a wide range of neurological and systemic conditions, suggesting their potential as early indicators of intracranial pressure and neurofluid imbalance. Nonetheless, several aspects of these spaces are still controversial. This article offers an overview of the current knowledge and magnetic resonance imaging characteristics of peri- and para-vascular spaces, which can help in daily clinical practice image description and interpretation. This paper is organized into different sections, including the microscopic anatomy of peri- and para-vascular spaces, their associations with pathological and physiological events, and their differential diagnosis.
Keywords: MRI; PVS; Virchow–Robin spaces; brain cysts; glymphatic system; magnetic resonance imaging; neurofluids; neuroradiology; paravascular spaces; perivascular spaces.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Robin C. Recherches Sur Quelques Particularites de La Structure Des Capillaires de l’encephale. J. Physiol. Homme Anim. 1859;2:537–548.
-
- Virchow R. Ueber die Erweiterung kleinerer Gefäfse. Arch. Für Pathol. Anat. Physiol. Für Klin. Med. 1851;3:427–462. doi: 10.1007/BF01960918. - DOI
-
- Iliff J.J., Wang M., Liao Y., Plogg B.A., Peng W., Gundersen G.A., Benveniste H., Vates G.E., Deane R., Goldman S.A., et al. A Paravascular Pathway Facilitates CSF Flow through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

