Pathogenesis of DJ-1/PARK7-Mediated Parkinson's Disease
- PMID: 38391909
- PMCID: PMC10887164
- DOI: 10.3390/cells13040296
Pathogenesis of DJ-1/PARK7-Mediated Parkinson's Disease
Abstract
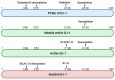
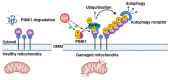
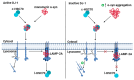
Parkinson's disease (PD) is a common movement disorder associated with the degeneration of dopaminergic neurons in the substantia nigra pars compacta. Mutations in the PD-associated gene PARK7 alter the structure and function of the encoded protein DJ-1, and the resulting autosomal recessively inherited disease increases the risk of developing PD. DJ-1 was first discovered in 1997 as an oncogene and was associated with early-onset PD in 2003. Mutations in DJ-1 account for approximately 1% of all recessively inherited early-onset PD occurrences, and the functions of the protein have been studied extensively. In healthy subjects, DJ-1 acts as an antioxidant and oxidative stress sensor in several neuroprotective mechanisms. It is also involved in mitochondrial homeostasis, regulation of apoptosis, chaperone-mediated autophagy (CMA), and dopamine homeostasis by regulating various signaling pathways, transcription factors, and molecular chaperone functions. While DJ-1 protects neurons against damaging reactive oxygen species, neurotoxins, and mutant α-synuclein, mutations in the protein may lead to inefficient neuroprotection and the progression of PD. As current therapies treat only the symptoms of PD, the development of therapies that directly inhibit oxidative stress-induced neuronal cell death is critical. DJ-1 has been proposed as a potential therapeutic target, while oxidized DJ-1 could operate as a biomarker for PD. In this paper, we review the role of DJ-1 in the pathogenesis of PD by highlighting some of its key neuroprotective functions and the consequences of its dysfunction.
Keywords: DJ-1; PARK7; Parkinson’s disease; mitochondria; neuroprotection; neurotoxicity; oxidative stress; reactive oxygen species.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

