Association between Donor Age and Osteogenic Potential of Human Adipose Stem Cells in Bone Tissue Engineering
- PMID: 38392210
- PMCID: PMC10887920
- DOI: 10.3390/cimb46020092
Association between Donor Age and Osteogenic Potential of Human Adipose Stem Cells in Bone Tissue Engineering
Abstract
Adipose stem cells (ASCs) have multilineage differentiation capacity and hold great potential for regenerative medicine. Compared to bone marrow-derived mesenchymal stem cells (bmMSCs), ASCs are easier to isolate from abundant sources with significantly higher yields. It is generally accepted that bmMSCs show age-related changes in their proliferation and differentiation potentials, whereas this aspect is still controversial in the case of ASCs. In this review, we evaluated the existing data on the effect of donor age on the osteogenic potential of human ASCs. Overall, a poor agreement has been achieved because of inconsistent findings in the previous studies. Finally, we attempted to delineate the possible reasons behind the lack of agreements reported in the literature. ASCs represent a heterogeneous cell population, and the osteogenic potential of ASCs can be influenced by donor-related factors such as age, but also gender, lifestyle, and the underlying health and metabolic state of donors. Furthermore, future studies should consider experimental factors in in vitro conditions, including passaging, cryopreservation, culture conditions, variations in differentiation protocols, and readout methods.
Keywords: ASC; aging; osteoblast; osteogenic potential; regenerative medicine; tissue engineering.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
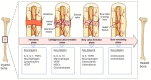

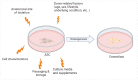
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

