Friend or Foe: Protein Inhibitors of DNA Gyrase
- PMID: 38392303
- PMCID: PMC10886550
- DOI: 10.3390/biology13020084
Friend or Foe: Protein Inhibitors of DNA Gyrase
Abstract
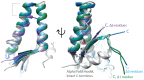
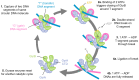
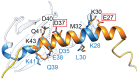
DNA gyrase is essential for the successful replication of circular chromosomes, such as those found in most bacterial species, by relieving topological stressors associated with unwinding the double-stranded genetic material. This critical central role makes gyrase a valued target for antibacterial approaches, as exemplified by the highly successful fluoroquinolone class of antibiotics. It is reasonable that the activity of gyrase could be intrinsically regulated within cells, thereby helping to coordinate DNA replication with doubling times. Numerous proteins have been identified to exert inhibitory effects on DNA gyrase, although at lower doses, it can appear readily reversible and therefore may have regulatory value. Some of these, such as the small protein toxins found in plasmid-borne addiction modules, can promote cell death by inducing damage to DNA, resulting in an analogous outcome as quinolone antibiotics. Others, however, appear to transiently impact gyrase in a readily reversible and non-damaging mechanism, such as the plasmid-derived Qnr family of DNA-mimetic proteins. The current review examines the origins and known activities of protein inhibitors of gyrase and highlights opportunities to further exert control over bacterial growth by targeting this validated antibacterial target with novel molecular mechanisms. Furthermore, we are gaining new insights into fundamental regulatory strategies of gyrase that may prove important for understanding diverse growth strategies among different bacteria.
Keywords: DNA gyrase; GyrA; GyrB; antibiotics; gyrase inhibitors; gyrase regulation; mobile genetic elements; quinolones; target protection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Ciprofloxacin and the fluoroquinolones. New concepts on the mechanism of action and resistance.Am J Med. 1989 Nov 30;87(5A):2S-8S. doi: 10.1016/0002-9343(89)90010-7. Am J Med. 1989. PMID: 2574005
-
Mechanism of plasmid-mediated quinolone resistance.Proc Natl Acad Sci U S A. 2002 Apr 16;99(8):5638-42. doi: 10.1073/pnas.082092899. Epub 2002 Apr 9. Proc Natl Acad Sci U S A. 2002. PMID: 11943863 Free PMC article.
-
Fluoroquinolone-gyrase-DNA complexes: two modes of drug binding.J Biol Chem. 2014 May 2;289(18):12300-12. doi: 10.1074/jbc.M113.529164. Epub 2014 Feb 4. J Biol Chem. 2014. PMID: 24497635 Free PMC article.
-
Mycobacterium tuberculosis DNA gyrase as a target for drug discovery.Infect Disord Drug Targets. 2007 Jun;7(2):159-68. doi: 10.2174/187152607781001763. Infect Disord Drug Targets. 2007. PMID: 17970226 Review.
-
Mode of action of the quinolone antimicrobial agents: review of recent information.Rev Infect Dis. 1989 Jul-Aug;11 Suppl 5:S902-11. doi: 10.1093/clinids/11.supplement_5.s902. Rev Infect Dis. 1989. PMID: 2549608 Review.
Cited by
-
Temporospatial control of topoisomerases by essential cellular processes.Curr Opin Microbiol. 2024 Dec;82:102559. doi: 10.1016/j.mib.2024.102559. Epub 2024 Nov 8. Curr Opin Microbiol. 2024. PMID: 39520813 Review.
-
Broken beyond repair: TA system ParE toxins mediate effective gyrase inhibition without driving resistance.J Bacteriol. 2025 Mar 20;207(3):e0041624. doi: 10.1128/jb.00416-24. Epub 2025 Mar 3. J Bacteriol. 2025. PMID: 40029095 Free PMC article.
-
Comprehensive genome analysis of Mycobacterium avium subsp. paratuberculosis in camels from Saudi Arabia: Molecular epidemiology and antimicrobial resistance.Vet World. 2025 Apr;18(4):859-876. doi: 10.14202/vetworld.2025.859-876. Epub 2025 Apr 19. Vet World. 2025. PMID: 40453949 Free PMC article.
-
Psychiatric disorders associated with fluoroquinolones: a pharmacovigilance analysis of the FDA adverse event reporting system database.Front Pharmacol. 2024 Oct 14;15:1435923. doi: 10.3389/fphar.2024.1435923. eCollection 2024. Front Pharmacol. 2024. PMID: 39469624 Free PMC article.
-
Corallocarpus glomeruliflorus: Pharmacological potential revealed by phytochemical and in silico investigations.Biochem Biophys Rep. 2025 Feb 7;41:101940. doi: 10.1016/j.bbrep.2025.101940. eCollection 2025 Mar. Biochem Biophys Rep. 2025. PMID: 39995632 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

