Evaluation of Tacrolimus' Adverse Effects on Zebrafish in Larval and Adult Stages by Using Multiple Physiological and Behavioral Endpoints
- PMID: 38392330
- PMCID: PMC10886482
- DOI: 10.3390/biology13020112
Evaluation of Tacrolimus' Adverse Effects on Zebrafish in Larval and Adult Stages by Using Multiple Physiological and Behavioral Endpoints
Abstract
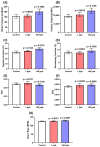
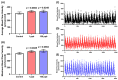
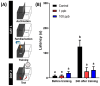
Tacrolimus (FK506) is a common immunosuppressant that is used in organ transplantation. However, despite its importance in medical applications, it is prone to adverse side effects. While some studies have demonstrated its toxicities to humans and various animal models, very few studies have addressed this issue in aquatic organisms, especially zebrafish. Here, we assessed the adverse effects of acute and chronic exposure to tacrolimus in relatively low doses in zebrafish in both larval and adult stages, respectively. Based on the results, although tacrolimus did not cause any cardiotoxicity and respiratory toxicity toward zebrafish larvae, it affected their locomotor activity performance in light-dark locomotion tests. Meanwhile, tacrolimus was also found to slightly affect the behavior performance, shoaling formation, circadian rhythm locomotor activity, and color preference of adult zebrafish in a dose-dependent manner. In addition, alterations in the cognitive performance of the fish were also displayed by the treated fish, indicated by a loss of short-term memory. To help elucidate the toxicity mechanism of tacrolimus, molecular docking was conducted to calculate the strength of the binding interaction between tacrolimus to human FKBP12. The results showed a relatively normal binding affinity, indicating that this interaction might only partly contribute to the observed alterations. Nevertheless, the current research could help clinicians and researchers to further understand the toxicology of tacrolimus, especially to zebrafish, thus highlighting the importance of considering the toxicity of tacrolimus prior to its usage.
Keywords: behaviors; memory; tacrolimus; toxicity; zebrafish.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









Similar articles
-
Evaluation of sub-chronic toxicity of melamine via systematic or oral delivery in adult zebrafish based on behavioral endpoints.Neurotoxicology. 2024 May;102:68-80. doi: 10.1016/j.neuro.2024.04.003. Epub 2024 Apr 9. Neurotoxicology. 2024. PMID: 38599288
-
A Novel Function of the Lysophosphatidic Acid Receptor 3 (LPAR3) Gene in Zebrafish on Modulating Anxiety, Circadian Rhythm Locomotor Activity, and Short-Term Memory.Int J Mol Sci. 2020 Apr 18;21(8):2837. doi: 10.3390/ijms21082837. Int J Mol Sci. 2020. PMID: 32325720 Free PMC article.
-
Evaluation of the Adverse Effects of Chronic Exposure to Donepezil (An Acetylcholinesterase Inhibitor) in Adult Zebrafish by Behavioral and Biochemical Assessments.Biomolecules. 2020 Sep 18;10(9):1340. doi: 10.3390/biom10091340. Biomolecules. 2020. PMID: 32962160 Free PMC article.
-
The agrochemical S-metolachlor disrupts molecular mediators and morphology of the swim bladder: Implications for locomotor activity in zebrafish (Danio rerio).Ecotoxicol Environ Saf. 2021 Jan 15;208:111641. doi: 10.1016/j.ecoenv.2020.111641. Epub 2020 Nov 19. Ecotoxicol Environ Saf. 2021. PMID: 33396161
-
Chronic Exposure to Low Concentration Lead Chloride-Induced Anxiety and Loss of Aggression and Memory in Zebrafish.Int J Mol Sci. 2020 Mar 7;21(5):1844. doi: 10.3390/ijms21051844. Int J Mol Sci. 2020. PMID: 32156000 Free PMC article.
References
-
- Jewett A., Tseng H.-C. In: Pharmacology and Therapeutics for Dentistry. 7th ed. Mariotti A., Dowd F.J., Johnson B.S., editors. Elsevier Health Sciences; St. Louis, MI, USA: 2017.
-
- McCulloh J.R. In: Infectious Diseases. 4th ed. Cohen J., Powderly W.G., Opal S.M., editors. Elsevier Health Sciences; Amsterdam, The Netherlands: 2017.
LinkOut - more resources
Full Text Sources

