Structural and Functional Insights into CRF Peptides and Their Receptors
- PMID: 38392338
- PMCID: PMC10886364
- DOI: 10.3390/biology13020120
Structural and Functional Insights into CRF Peptides and Their Receptors
Abstract
Corticotropin-releasing factor or hormone (CRF or CRH) and the urocortins regulate a plethora of physiological functions and are involved in many pathophysiological processes. CRF and urocortins belong to the family of CRF peptides (CRF family), which includes sauvagine, urotensin, and many synthetic peptide and non-peptide CRF analogs. Several of the CRF analogs have shown considerable therapeutic potential in the treatment of various diseases. The CRF peptide family act by interacting with two types of plasma membrane proteins, type 1 (CRF1R) and type 2 (CRF2R), which belong to subfamily B1 of the family B G-protein-coupled receptors (GPCRs). This work describes the structure of CRF peptides and their receptors and the activation mechanism of the latter, which is compared with that of other GPCRs. It also discusses recent structural information that rationalizes the selective binding of various ligands to the two CRF receptor types and the activation of receptors by different agonists.
Keywords: CRF-peptides; CRF-receptors; activation; agonists; antagonists; binding; structure.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

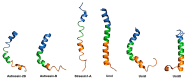
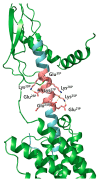

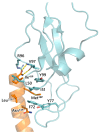
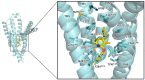
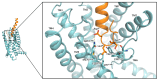
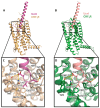
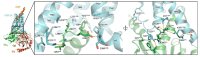
Similar articles
-
Determinants of corticotropin releasing factor. Receptor selectivity of corticotropin releasing factor related peptides.J Med Chem. 2004 Jun 17;47(13):3450-4. doi: 10.1021/jm049883l. J Med Chem. 2004. PMID: 15189041
-
Understanding Corticotropin Releasing Factor Receptor (CRFR) Activation Using Structural Models.Curr Mol Pharmacol. 2017;10(4):325-333. doi: 10.2174/1874467210666170110122939. Curr Mol Pharmacol. 2017. PMID: 28103783 Review.
-
Molecular Basis for Hormone Recognition and Activation of Corticotropin-Releasing Factor Receptors.Mol Cell. 2020 Feb 6;77(3):669-680.e4. doi: 10.1016/j.molcel.2020.01.013. Epub 2020 Jan 30. Mol Cell. 2020. PMID: 32004470
-
Exploring the binding site crevice of a family B G protein-coupled receptor, the type 1 corticotropin releasing factor receptor.Mol Pharmacol. 2010 Oct;78(4):785-93. doi: 10.1124/mol.110.065474. Epub 2010 Jul 27. Mol Pharmacol. 2010. PMID: 20664003
-
Evolution and physiology of the corticotropin-releasing factor (CRF) family of neuropeptides in vertebrates.Gen Comp Endocrinol. 1999 Jul;115(1):1-22. doi: 10.1006/gcen.1999.7298. Gen Comp Endocrinol. 1999. PMID: 10375459 Review.
Cited by
-
Urocortin2 measurement for heart failure assessment.Sci Rep. 2025 Apr 24;15(1):14381. doi: 10.1038/s41598-025-99509-4. Sci Rep. 2025. PMID: 40275076 Free PMC article.
References
-
- Reyes T.M., Lewis K., Perrin M.H., Kunitake K.S., Vaughan J., Arias C.A., Hogenesch J.B., Gulyas J., Rivier J., Vale W.W., et al. Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc. Natl. Acad. Sci. USA. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

