Solvation Thermodynamics and Its Applications
- PMID: 38392429
- PMCID: PMC10887854
- DOI: 10.3390/e26020174
Solvation Thermodynamics and Its Applications
Abstract
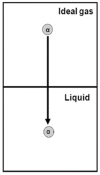
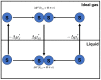
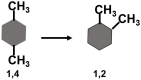
In this article, we start by describing a few "definitions" of the solvation processes, which were used in the literature until about 1980. Then, we choose one of these definitions and show that it has a simple molecular interpretation. This fact led to a new definition of the solvation process and the corresponding thermodynamic quantities. The new measure of the solvation Gibbs energy has a simple interpretation. In addition, the thermodynamic quantities associated with the new solvation process have several other advantages over the older measures. These will be discussed briefly in the third section. In the fourth section, we discuss a few applications of the new solvation process.
Keywords: Gibbs energy of solvation; solvation; thermodynamics.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
-
- Ben-Naim A. Solvation Thermodynamics. Plenum Press; New York, NY, USA: Springer; Berlin/Heidelberg, Germany: 1987.
-
- Gurney R.W. Ionic Processes in Solution. Dover Publication; New York, NY, USA: 1953.
-
- Encyclopedia Britannica. 5th ed. Encyclopædia Britannica, Inc.; Chicago, IL, USA: 1974. p. 1055.
-
- Denbigh K.G. The Principle of Chemical Equilibrium. Cambridge University Press; London, UK: 1966.
-
- Tanford C. The Hydrophobic Effect. John Wiley; New York, NY, USA: 1973.
Publication types
LinkOut - more resources
Full Text Sources

