The Extraordinary Diversity of Merodon avidus Complex (Diptera: Syrphidae)-Adding New Areas, New Species and a New Molecular Marker
- PMID: 38392524
- PMCID: PMC10888622
- DOI: 10.3390/insects15020105
The Extraordinary Diversity of Merodon avidus Complex (Diptera: Syrphidae)-Adding New Areas, New Species and a New Molecular Marker
Abstract
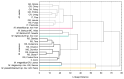
In this paper, the Merodon avidus (Diptera, Syrphidae) species complex was revised, whereupon we discovered and described four new species for science: Merodon atroavidus Vujić, Radenković et Likov sp. nov., M. magnus Vujić, Kočiš Tubić et Ačanski sp. nov., M. nigroscutum Vujić, Radenković et Likov sp. nov. and M. pseudomoenium Vujić, Kočiš Tubić et Ačanski sp. nov. An integrative taxonomy approach was used to delimit species boundaries. Two molecular markers (the mitochondrial COI gene and nuclear 28S rRNA gene-newly analysed marker for the complex) and geometric morphometry of the wing shape, together with morphological data and distribution, successfully separated all species from the complex. The morphological variability of the analysed species is described and discussed and an illustrated diagnostic key for typical morpho-forms of species from the M. avidus complex is presented. A distribution map of all investigated species from the complex is provided. The level of endemicity of the M. avidus complex was discussed.
Keywords: 28S rRNA gene; COI gene; distribution; hoverflies; taxonomy; wing shape.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




















References
-
- Vujić A., Tot T., Andrić A., Ačanski J., Zorić L.Š., Pérez-Bañón C., Aracil A., Veselić S., Arok M., Mengual Sanchis X., et al. Review of the Merodon natans group with description of a new species, a key to the adults of known species of the natans lineage and first descriptions of some preimaginal stages. Arthropod Syst. Phylogeny. 2021;79:343–378. doi: 10.3897/asp.79.e65861. - DOI
-
- Speight M.C.D. Species Accounts of European Syrphidae, 2020. Syrph the Net, the Database of European Syrphidae (Diptera) Syrph the Net Publications; Dublin, Ireland: 2020.
-
- Vujić A., Radenković S., Ståhls G., Ačanski J., Stefanović A., Veselić S., Andrić A., Hayat R. Systematics and taxonomy of the ruficornis group of genus Merodon (Diptera: Syrphidae) Syst. Entomol. 2012;37:578–602. doi: 10.1111/j.1365-3113.2012.00631.x. - DOI
-
- Vujić A., Radenković S., Kočiš Tubić N., Likov L., Popov G., Rojo S., Miličić M. Integrative taxonomy of the Merodon aberrans (Diptera, Syrphidae) species group: Distribution patterns and description of three new species. Contrib. Zool. 2022;92:51–96. doi: 10.1163/18759866-bja10037. - DOI
Grants and funding
- 451-03-47/2023-01/200125/Ministry of Science, Technological Development and Innovation of the Republic of Serbia
- 451-03-47/2023-01/200358/Ministry of Science, Technological Development and Innovation of the Republic of Serbia
- 142-451-3143/2022-01/2/Provincial Secretariat for Higher Education and Scientific Research of Autonomous Province of Vojvodina
- 7737504/Science Fund of the Republic of Serbia
- 739570/H2020 Project
LinkOut - more resources
Full Text Sources

