Investigating Efficient Risk-Stratified Pathways for the Early Detection of Clinically Significant Prostate Cancer
- PMID: 38392564
- PMCID: PMC10890536
- DOI: 10.3390/jpm14020130
Investigating Efficient Risk-Stratified Pathways for the Early Detection of Clinically Significant Prostate Cancer
Abstract
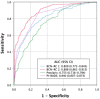
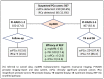
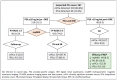
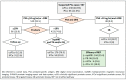
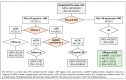
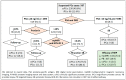
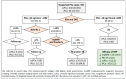
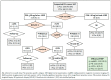
Risk-stratified pathways (RSPs) are recommended by the European Association of Uro-logy (EAU) to improve the early detection of clinically significant prostate cancer (csPCa). RSPs can reduce magnetic resonance imaging (MRI) demand, prostate biopsies, and the over-detection of insignificant PCa (iPCa). Our goal is to analyze the efficacy and cost-effectiveness of several RSPs by using sequential stratifications from the serum prostate-specific antigen level and digital rectal examination, the Barcelona risk calculators (BCN-RCs), MRI, and Proclarix™. In a cohort of 567 men with a serum PSA level above 3.0 ng/mL who underwent multiparametric MRI (mpMRI) and targeted and/or systematic biopsies, the risk of csPCa was retrospectively assessed using Proclarix™ and BCN-RCs 1 and 2. Six RSPs were compared with those recommended by the EAU that, stratifying men from MRI, avoided 16.7% of prostate biopsies with a prostate imaging-reporting and data system score of <3, with 2.6% of csPCa cases remaining undetected. The most effective RSP avoided mpMRI exams in men with a serum PSA level of >10 ng/mL and suspicious DRE, following stratifications from BCN-RC 1, mpMRI, and Proclarix™. The demand for mpMRI decreased by 19.9%, prostate biopsies by 19.8%, and over-detection of iPCa by 22.7%, while 2.6% of csPCa remained undetected as in the recommended RSP. Cost-effectiveness remained when the Proclarix™ price was assumed to be below EUR 200.
Keywords: Barcelona risk calculator; Proclarix; prostate cancer; risk-stratified pathway; screening.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Reducing the demand for magnetic resonance imaging scans and prostate biopsies during the early detection of clinically significant prostate cancer: Applying the Barcelona risk-stratified pathway in Catalonia.Urol Oncol. 2024 Apr;42(4):115.e1-115.e7. doi: 10.1016/j.urolonc.2023.09.020. Epub 2024 Feb 10. Urol Oncol. 2024. PMID: 38342654
-
The Efficacy of Proclarix to Select Appropriate Candidates for Magnetic Resonance Imaging and Derived Prostate Biopsies in Men with Suspected Prostate Cancer.World J Mens Health. 2022 Apr;40(2):270-279. doi: 10.5534/wjmh.210117. Epub 2021 Dec 27. World J Mens Health. 2022. PMID: 35021312 Free PMC article.
-
Comparison of Rotterdam and Barcelona Magnetic Resonance Imaging Risk Calculators for Predicting Clinically Significant Prostate Cancer.Eur Urol Open Sci. 2023 May 22;53:46-54. doi: 10.1016/j.euros.2023.03.013. eCollection 2023 Jul. Eur Urol Open Sci. 2023. PMID: 37441350 Free PMC article.
-
A Clinically Significant Prostate Cancer Predictive Model Using Digital Rectal Examination Prostate Volume Category to Stratify Initial Prostate Cancer Suspicion and Reduce Magnetic Resonance Imaging Demand.Cancers (Basel). 2022 Oct 18;14(20):5100. doi: 10.3390/cancers14205100. Cancers (Basel). 2022. PMID: 36291883 Free PMC article.
-
Proclarix, A New Biomarker for the Diagnosis of Clinically Significant Prostate Cancer: A Systematic Review.Mol Diagn Ther. 2022 May;26(3):273-281. doi: 10.1007/s40291-022-00584-4. Epub 2022 Apr 26. Mol Diagn Ther. 2022. PMID: 35471698
Cited by
-
Validation of the Barcelona-MRI predictive model when PI-RADS v2.1 is used with trans-perineal prostate biopsies.Int Braz J Urol. 2024 Sep-Oct;50(5):595-604. doi: 10.1590/S1677-5538.IBJU.2024.0204. Int Braz J Urol. 2024. PMID: 39106115 Free PMC article.
-
Validation of the Barcelona Magnetic Resonance Imaging Predictive Model for Significant Prostate Cancer Detection in Men Undergoing Mapping per 0.5 Mm-Core Targeted Biopsies of Suspicious Lesions and Perilesional Areas.Cancers (Basel). 2025 Jan 31;17(3):473. doi: 10.3390/cancers17030473. Cancers (Basel). 2025. PMID: 39941840 Free PMC article.
References
-
- Catalona W.J., Richie J.P., Ahmann F.R., Hudson M.A., Scardino P.T., Flanigan R.C., DeKernion J.B., Ratliff T.L., Kavoussi L.R., Dalkin B.L., et al. Comparison of Digital Rectal Examination and Serum Prostate Specific Antigen in the Early Detection of Prostate Cancer: Results of a Multicenter Clinical Trial of 6,630 Men. J. Urol. 1994;151:1283–1290. doi: 10.1016/S0022-5347(17)35233-3. - DOI - PubMed
-
- Van Poppel H., Roobol M.J., Chapple C.R., Catto J.W.F., N’Dow J., Sønksen J., Stenzl A., Wirth M. Prostate-specific Antigen. Testing as Part. of a Risk-Adapted Early Detection Strategy for Prostate Cancer: European Association of Urology Position and Recommendations for 2021. Eur. Urol. 2021;80:703–711. doi: 10.1016/j.eururo.2021.07.024. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

