The Effect of Substrate Properties on Cellular Behavior and Nanoparticle Uptake in Human Fibroblasts and Epithelial Cells
- PMID: 38392715
- PMCID: PMC10892529
- DOI: 10.3390/nano14040342
The Effect of Substrate Properties on Cellular Behavior and Nanoparticle Uptake in Human Fibroblasts and Epithelial Cells
Abstract
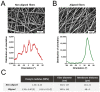
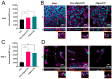
The delivery of nanomedicines into cells holds enormous therapeutic potential; however little is known regarding how the extracellular matrix (ECM) can influence cell-nanoparticle (NP) interactions. Changes in ECM organization and composition occur in several pathophysiological states, including fibrosis and tumorigenesis, and may contribute to disease progression. We show that the physical characteristics of cellular substrates, that more closely resemble the ECM in vivo, can influence cell behavior and the subsequent uptake of NPs. Electrospinning was used to create two different substrates made of soft polyurethane (PU) with aligned and non-aligned nanofibers to recapitulate the ECM in two different states. To investigate the impact of cell-substrate interaction, A549 lung epithelial cells and MRC-5 lung fibroblasts were cultured on soft PU membranes with different alignments and compared against stiff tissue culture plastic (TCP)/glass. Both cell types could attach and grow on both PU membranes with no signs of cytotoxicity but with increased cytokine release compared with cells on the TCP. The uptake of silica NPs increased more than three-fold in fibroblasts but not in epithelial cells cultured on both membranes. This study demonstrates that cell-matrix interaction is substrate and cell-type dependent and highlights the importance of considering the ECM and tissue mechanical properties when designing NPs for effective cell targeting and treatment.
Keywords: epithelial cells; extracellular matrix; fibroblasts; mechanobiology; nanofibers; nanoparticle uptake; stiffness.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Zhao J., Stenzel M.H. Entry of nanoparticles into cells: The importance of nanoparticle properties. Polym. Chem. 2018;9:259–272. doi: 10.1039/C7PY01603D. - DOI
-
- Engin A.B., Nikitovic D., Neagu M., Henrich-Noack P., Docea A.O., Shtilman M.I., Golokhvast K., Tsatsakis A.M. Mechanistic understanding of nanoparticles’ interactions with extracellular matrix: The cell and immune system. Part. Fibre Toxicol. 2017;14:22. doi: 10.1186/s12989-017-0199-z. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

