Best Practice Guidelines for the Management of Patients with Post-Stroke Spasticity: A Modified Scoping Review
- PMID: 38393176
- PMCID: PMC10892074
- DOI: 10.3390/toxins16020098
Best Practice Guidelines for the Management of Patients with Post-Stroke Spasticity: A Modified Scoping Review
Abstract
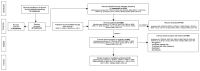
This article aims to provide a concise overview of the best available evidence for managing post-stroke spasticity. A modified scoping review, conducted following the PRISMA guidelines and the PRISMA Extension for Scoping Reviews (PRISMA-ScR), involved an intensive search on Medline and PubMed from 1 January 2000 to 31 August 2023. The focus was placed on high-quality (GRADE A) medical, rehabilitation, and surgical interventions. In total, 32 treatments for post-stroke spasticity were identified. Two independent reviewers rigorously assessed studies, extracting data, and evaluating bias using GRADE criteria. Only interventions with GRADE A evidence were considered. The data included the study type, number of trials, participant characteristics, interventions, parameters, controls, outcomes, and limitations. The results revealed eleven treatments supported by GRADE A evidence, comprising 14 studies. Thirteen were systematic reviews and meta-analyses, and one was randomized control trial. The GRADE A treatments included stretching exercises, static stretching with positional orthosis, transcutaneous electrical nerve stimulation, extracorporeal shock wave therapy, peripheral magnetic stimulation, non-invasive brain stimulation, botulinum toxin A injection, dry needling, intrathecal baclofen, whole body vibration, and localized muscle vibration. In conclusion, this modified scoping review highlights the multimodal treatments supported by GRADE A evidence as being effective for improving functional recovery and quality of life in post-stroke spasticity. Further research and exploration of new therapeutic options are encouraged.
Keywords: GRADE A; a modified scoping review; best evidence; multimodality treatments; post-stroke spasticity.
Conflict of interest statement
A.S., S.C. and C.C.P.C declare that they have no affiliations with or involvement in any organization or entity with any financial interests in the subject matter or materials discussed in this manuscript. D.M.S received research grants and consultancy honoraria from Allergan, Abbvie Company, Merz, and Ipsen.
Figures

References
-
- Ashford S., Turner-Stokes L., Allison R., Duke L., Moore P., Bavikatte G., Kirker S., Moore P., Ward A.B., Bilton D., et al. Spasticity in Adults: Management Using Botulinum Toxin. 2nd ed. The Royal College of Physicians; London, UK: 2018. National Guidelines.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

