Boron Compounds Mitigate 2,3,7,8-Tetrachlorodibenzo-p-dioxin-Induced Toxicity in Human Peripheral Blood Mononuclear Cells
- PMID: 38393193
- PMCID: PMC10891549
- DOI: 10.3390/toxics12020098
Boron Compounds Mitigate 2,3,7,8-Tetrachlorodibenzo-p-dioxin-Induced Toxicity in Human Peripheral Blood Mononuclear Cells
Abstract
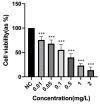
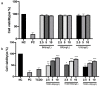
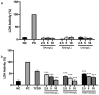
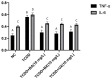
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) stands as one of the most potent halogenated polycyclic hydrocarbons, known to inflict substantial cytotoxic effects on both animal and human tissues. Its widespread presence and recalcitrance make it an environmental and health concern. Efforts are being intensively channeled to uncover strategies that could mitigate the adverse health outcomes associated with TCDD exposure. In the realm of counteractive agents, boron compounds are emerging as potential candidates. These compounds, which have found applications in a spectrum of industries ranging from agriculture to pharmaceutical and cosmetic manufacturing, are known to modulate several cellular processes and enzymatic pathways. However, the dose-response relationships and protective potentials of commercially prevalent boron compounds, such as boric acid (BA), ulexite (UX), and borax (BX), have not been comprehensively studied. In our detailed investigation, when peripheral blood mononuclear cells (PBMCs) were subjected to TCDD exposure, they manifested significant cellular disruptions. This was evidenced by compromised membrane integrity, a marked reduction in antioxidant defense mechanisms, and a surge in the malondialdehyde (MDA) levels, a recognized marker for oxidative stress. On the genomic front, increased 8-OH-dG levels and chromosomal aberration (CA) frequency suggested that TCDD had the potential to cause DNA damage. Notably, our experiments have revealed that boron compounds could act as protective agents against these disruptions. They exhibited a pronounced ability to diminish the cytotoxic, genotoxic, and oxidative stress outcomes instigated by TCDD. Thus, our findings shed light on the promising role of boron compounds. In specific dosages, they may not only counteract the detrimental effects of TCDD but also serve as potential chemopreventive agents, safeguarding the cellular and genomic integrity of PBMCs.
Keywords: TCDD; boron compounds; cytotoxicity; genotoxicity; oxidative status.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Sha R., Chen Y., Wang Y., Luo Y., Liu Y., Ma Y., Li Y., Xu L., Xie H.Q., Zhao B. Gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice: Neurobehavioral effects on female offspring. Sci. Total Environ. 2021;752:141784. doi: 10.1016/j.scitotenv.2020.141784. - DOI - PubMed
-
- Faiad W., Soukkarieh C., Hanano A. 2,3,7,8-tetrachlorodibenzo-p-dioxin induces multigenerational testicular toxicity and biosynthetic disorder of testosterone in BALB/C mice: Transcriptional, histopathological and hormonal determinants. Ecotoxicol. Environ. Saf. 2023;263:115233. doi: 10.1016/j.ecoenv.2023.115233. - DOI - PubMed
LinkOut - more resources
Full Text Sources

