Piezo1 mechanosensing regulates integrin-dependent chemotactic migration in human T cells
- PMID: 38393325
- PMCID: PMC10942591
- DOI: 10.7554/eLife.91903
Piezo1 mechanosensing regulates integrin-dependent chemotactic migration in human T cells
Abstract
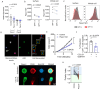
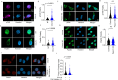
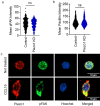
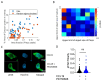
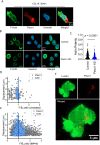
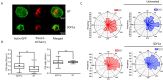
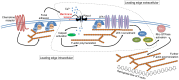
T cells are crucial for efficient antigen-specific immune responses and thus their migration within the body, to inflamed tissues from circulating blood or to secondary lymphoid organs, plays a very critical role. T cell extravasation in inflamed tissues depends on chemotactic cues and interaction between endothelial adhesion molecules and cellular integrins. A migrating T cell is expected to sense diverse external and membrane-intrinsic mechano-physical cues, but molecular mechanisms of such mechanosensing in cell migration are not established. We explored if the professional mechanosensor Piezo1 plays any role during integrin-dependent chemotaxis of human T cells. We found that deficiency of Piezo1 in human T cells interfered with integrin-dependent cellular motility on ICAM-1-coated surface. Piezo1 recruitment at the leading edge of moving T cells is dependent on and follows focal adhesion formation at the leading edge and local increase in membrane tension upon chemokine receptor activation. Piezo1 recruitment and activation, followed by calcium influx and calpain activation, in turn, are crucial for the integrin LFA1 (CD11a/CD18) recruitment at the leading edge of the chemotactic human T cells. Thus, we find that Piezo1 activation in response to local mechanical cues constitutes a membrane-intrinsic component of the 'outside-in' signaling in human T cells, migrating in response to chemokines, that mediates integrin recruitment to the leading edge.
Keywords: F-actin; LFA1; Piezo1; cell biology; cell migration; chemotaxis; confocal microscopy; focal adhesion kinase; human; human T cells; immunology; inflammation; interference reflection microscopy; mechanosensing.
© 2023, Liu et al.
Conflict of interest statement
CL, TM, PB, MH, PB, BS, JS, RD, DS, BS, DG No competing interests declared
Figures





Update of
- doi: 10.1101/2022.08.16.504114
- doi: 10.7554/eLife.91903.1
- doi: 10.7554/eLife.91903.2
References
-
- Atcha H, Jairaman A, Holt JR, Meli VS, Nagalla RR, Veerasubramanian PK, Brumm KT, Lim HE, Othy S, Cahalan MD, Pathak MM, Liu WF. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nature Communications. 2021;12:3256. doi: 10.1038/s41467-021-23482-5. - DOI - PMC - PubMed
-
- Chakraborty M, Mukherjee B, Nalinakshan N, Biswas A, Nayak RK, Sinha B. Effect of heterogeneous substrate adhesivity of follower cells on speed and tension profile of leader cells in primary keratocyte collective cell migration. Biology Open. 2022;11:bio058893. doi: 10.1242/bio.058893. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

