Impact of hemodialysis on efficacies of the antiplatelet agents in coronary artery disease patients complicated with end-stage renal disease
- PMID: 38393676
- PMCID: PMC11026285
- DOI: 10.1007/s11239-023-02924-5
Impact of hemodialysis on efficacies of the antiplatelet agents in coronary artery disease patients complicated with end-stage renal disease
Abstract
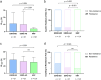
It is controversial whether hemodialysis affects the efficacy of the antiplatelet agents. We aimed to investigate the impact of hemodialysis on efficacies of the antiplatelet agents in coronary artery disease (CAD) patients complicated with end-stage renal disease (ESRD). 86 CAD patients complicated with ESRD requiring hemodialysis were consecutively enrolled. After 5-day treatment with aspirin and clopidogrel or ticagrelor, the platelet aggregations induced by arachidonic acid (PLAA) or adenosine diphosphate (PLADP), and the P2Y12 reaction unit (PRU) were measured before and after hemodialysis. The propensity matching score method was adopted to generate a control group with normal renal function from 2439 CAD patients. In patients taking aspirin, the PLAA remained unchanged after hemodialysis. In patients taking clopidogrel, the PLADP (37.26 ± 17.04 vs. 31.77 ± 16.09, p = 0.029) and corresponding clopidogrel resistance (CR) rate (23 [48.9%] vs. 14 [29.8%], p = 0.022) significantly decreased after hemodialysis, though PRU remained unchanged. Subgroup analysis indicated that PLADP significantly decreased while using polysulfone membrane (36.8 ± 17.9 vs. 31.1 ± 14.5, p = 0.024). In patients taking ticagrelor, PLADP, and PRU remained unchanged after hemodialysis. ESRD patients had higher incidences of aspirin resistance (AR) and CR compared to those with normal renal function (AR: 16.1% vs. 0%, p = 0.001; CR: 48.4% vs. 24.8%, p = 0.024). Hemodialysis does not have negative effect on the efficacies of aspirin, clopidogrel and ticagrelor in ESRD patients with CAD. ESRD patients have higher incidences of AR and CR compared with those with normal renal function.Trial registration ClinicalTrials.gov Identifier: NCT03330223, first registered January 4, 2018.
Keywords: Antiplatelet therapy; Coronary artery disease; End-stage renal disease; Hemodialysis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
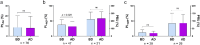

Similar articles
-
Platelet reactivity after receiving clopidogrel compared with ticagrelor in patients with kidney failure treated with hemodialysis: a randomized crossover study.Am J Kidney Dis. 2015 Jun;65(6):916-24. doi: 10.1053/j.ajkd.2014.11.023. Epub 2015 Jan 24. Am J Kidney Dis. 2015. PMID: 25622774 Clinical Trial.
-
Ticagrelor Versus Clopidogrel in Black Patients With Stable Coronary Artery Disease: Prospective, Randomized, Open-Label, Multiple-Dose, Crossover Pilot Study.Circ Cardiovasc Interv. 2015 Jul;8(7):e002232. doi: 10.1161/CIRCINTERVENTIONS.114.002232. Circ Cardiovasc Interv. 2015. PMID: 26152562 Clinical Trial.
-
Haemodialysis impairs clopidogrel but not aspirin responsiveness in patients with end-stage renal disease. Results of a pilot study.Thromb Haemost. 2014 Apr 1;111(4):662-9. doi: 10.1160/TH13-04-0289. Epub 2013 Dec 12. Thromb Haemost. 2014. PMID: 24337367
-
Ticagrelor: an investigational oral antiplatelet treatment for reduction of major adverse cardiac events in patients with acute coronary syndrome.Vasc Health Risk Manag. 2010 Oct 21;6:963-77. doi: 10.2147/VHRM.S13263. Vasc Health Risk Manag. 2010. PMID: 21057581 Free PMC article. Review.
-
Review of aspirin and clopidogrel resistance in peripheral arterial disease.J Vasc Surg. 2017 Nov;66(5):1576-1586. doi: 10.1016/j.jvs.2017.07.065. J Vasc Surg. 2017. PMID: 28893489 Review.
Cited by
-
Antithrombotic Therapy in Acute Coronary Syndrome Patients with End-Stage Renal Disease: Navigating Efficacy and Safety.J Clin Med. 2025 Jun 3;14(11):3956. doi: 10.3390/jcm14113956. J Clin Med. 2025. PMID: 40507717 Free PMC article. Review.
References
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

